
If it takes 11 minutes to cook spaghetti in Ann Arbor, Michigan, and 14 minutes in Boulder, Colorado, how long would it take in Cuzco, Peru? Discuss ways to make the spaghetti more tasty. If you prefer to make a creative spaghetti dinner for family or friends rather than answering this question, that’s OK, too; you’ll get full credit—but only if you turn in your recipe and bring your instructor a taste.
Interpretation:
The time takes to cook spaghetti in Cuzco, Peru should be calculated.
Concept Introduction:
The reaction in which its rate is constant and independent of reactant concentration is zero order reaction.
For zero order reaction the combined rate law and mole balance is as follows,
Answer to Problem 5.2Q
The time takes to cook spaghetti in Cuzco, Peru is
Explanation of Solution
Given:
In Ann Arbor, Michigan it takes 11 minutes to cook spaghetti and in Boulder, Colorado it takes 14 minutes.
The boiling point present in these regions are represented as follows,
Let’s assume that given reaction is zero order conversion, and then rate law is depicted as follows,
(For zero order reaction the rate constant
For complete conversion means the spaghetti is well cooked the
Hence plotting
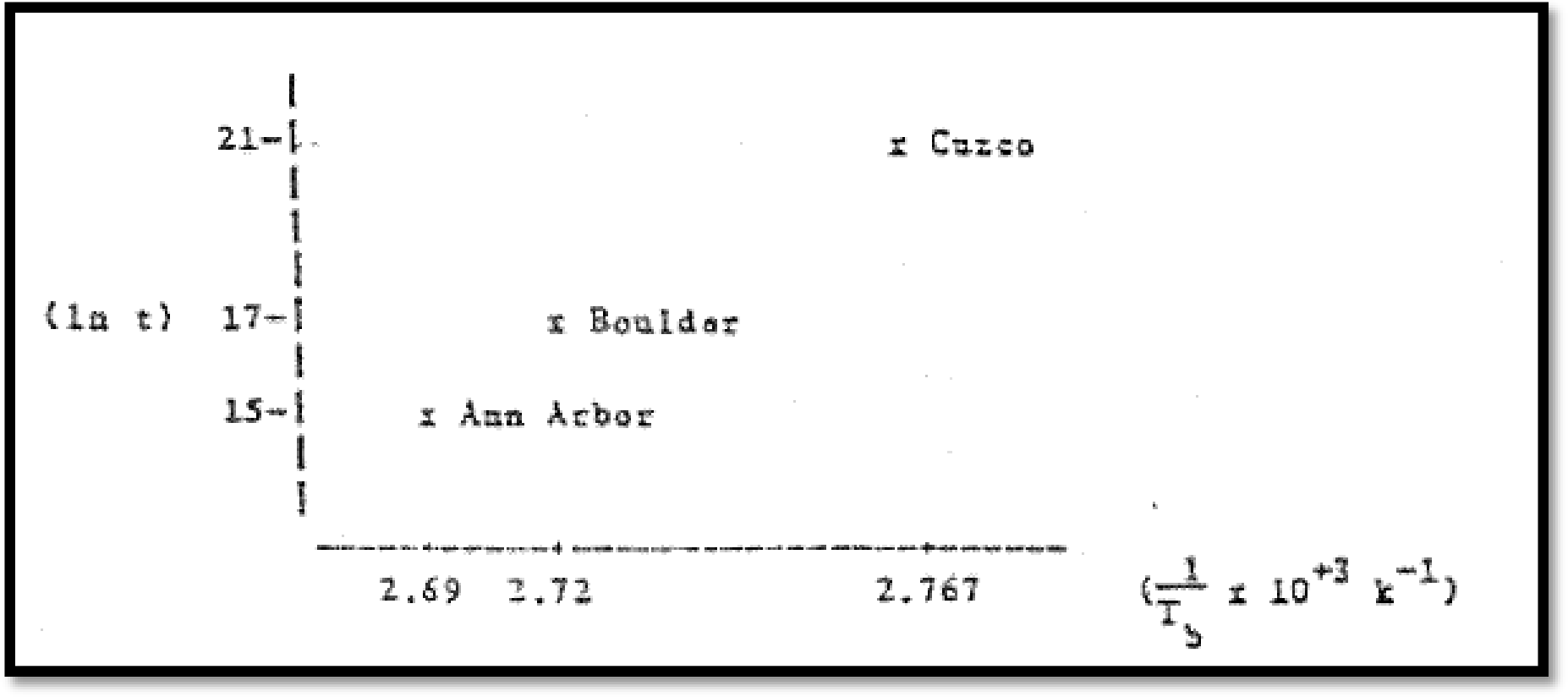
Therefore, the time takes to cook spaghetti in Cuzco, Peru is
The time takes to cook spaghetti in Cuzco, Peru was calculated.
Want to see more full solutions like this?
Chapter 5 Solutions
ELEMENTS OF CHEM. REACTION ENGR
Additional Engineering Textbook Solutions
Problem Solving with C++ (10th Edition)
Starting Out with Python (4th Edition)
Mechanics of Materials (10th Edition)
Starting Out with Java: From Control Structures through Objects (7th Edition) (What's New in Computer Science)
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Computer Science: An Overview (13th Edition) (What's New in Computer Science)
- Figure below shows a portion of a fire protection system in which apump draws water at 60 F from a reservoir and delivers it to point B at the flow rate of 1500 gal/min a). Calculate the required height of the water level in the tank in order to maintain 5.0 psig pressure at point A. Answer: h = 12,6 ft b). Assuming that the pressure at A is 5.0 psig, calculate the power delivered by the pump to the water in order to maintain the pressure at point B at 85 kPa. Include energy lost due to friction but neglect any other energy losses. P₁ =19,2 hparrow_forwardWater at 60° F is being pumped from a stream to a reservoir whose surface is 210 ft above the pump. The pipe from the pump to the reservoir is an 8-in Schedule 40 steel pipe 2500 ft long. The pressure at the pump inlet is - 2,36 psig. If 4.00 ft³/s is being pumped, a). Compute the pressure at the outlet of the pump. Answer: 0,997 MPa b). Compute the power delivered by the pump to the water. Answer: 151 hp Consider the friction loss in the discharged line, but neglect other lossesarrow_forward1. Consider a mixture of 2.5.0% ethane, 2.0% butane, and 1.7% n-pentane by volume.a. Estimate the LFL and UFL of the mixture. Is it flammable?b. Estimate the LOC for this mixture.arrow_forward
- Estimate the LFL and UFL for propylene using Equations 6-10 and 6-11 in the textbook,and compare these to the experimental values given in the table in Appendix B.arrow_forward1. Determine the minimum compression ratio required to raise the temperature of air overhexane to its AIT. Assume an initial temperature of 20°C.2. Ethanol is kept in a storage vessel that is vented with air (at 25°C and 1 atm). Is theequilibrium mixture of vapor above the liquid and air flammable? What if the liquid isacetone instead?arrow_forwardHydrogenation of Ethylbenzene to Styrene Reaction: C₈H₁₀ → C₈H₈ + H₂ΔHᵣ°(300°C) = -124 kJ/mol (exact value unknown) Process Description: The basis is 1000 kg/h of separated styrene. The reaction conversion rate is 35%. The temperature increase in heat exchanger 2 is adiabatic. A fresh stream of pure ethylbenzene (25°C) enters a mixing vessel, where it is combined with a recycle stream (from the distillation column, as explained later), which also consists of pure ethylbenzene at 25°C. After mixing, the stream is sent to a heat exchanger (HX1), where the mixture is heated to 200°C. Next, the mixture enters an adiabatic heat exchanger (HX2), where it is further heated to 300°C by adding steam (at 350°C). This steam is used to prevent side reactions and carbon deposition in the reactor. The heated mixture is then fed into the reactor, where the reaction takes place with a conversion rate of 35%. As a result, the mixture cools down to 260°C. The resulting mixture is then sent to HX4, where…arrow_forward
- Chemical Engineering Questionarrow_forward4.5arrow_forwardPhosphoric acid (H3PO4) is a triprotic acid. Na2HPO4 is added to deionized water at a concentration of 0.02 M. A. Write the mass balance for this solution B. Write the charge balance for this solution C. Write the proton condition for this solutionarrow_forward
- 4.10arrow_forward4.16 aarrow_forward8. The thermal decomposition of nitric oxide at elevated temperatures 2NO → N₂+02 has been studied in a batch reactor where at temperatures below 2000K the rate expression that applies to low conversions is: r = kCm05 Co At high conversions, or when the initial mixture contains a high concentration of O2 the rate expression is given by: r = k' Cм0.5 C15C0,5 To explain these kinetics the following chain reaction mechanism has been proposed: Initiation: Propagation: 2NON₂O +0 k₂ E1=272.0 kJ/mol 0+ NO O₂+ N E₂-161.0 kJ/mol N+NO N₂+0 E3-1.4 kJ/mol K4 20+ MO₂+M E4=14.0 kJ/mol ks Termination: where M is any molecule capable of the energy transfer necessary to stabilize the oxygen molecule. Once appreciable amounts of O2 are present in the reaction mixture, the initiation reaction that is the primary source of atomic oxygen is no longer the first reaction. Instead, the following reaction begins to dominate the chain initiation process: Initiation (high O2): ks NO +0₂ NO₂+0 E5=198.0 kJ/mol a.…arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





