
The Science and Engineering of Materials (MindTap Course List)
7th Edition
ISBN: 9781305076761
Author: Donald R. Askeland, Wendelin J. Wright
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 5.1P
What is the driving force for diffusion?
Expert Solution & Answer
To determine
The driving force for diffusion.
Explanation of Solution
Diffusion is the process of movement of substance from high concentration area to the low concentration area. Diffusion is more in fluid as compare to solid due to less intermolecular force and spacing.
Driving force for diffusion is the gradient of concentration with respect to distance which is expressed as 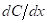 with the units of
with the units of 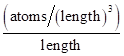 Diffusion flux does not depend on the time for steady state diffusion which is expressed as follows:
Diffusion flux does not depend on the time for steady state diffusion which is expressed as follows:
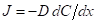
Here, 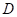 is the diffusion coefficient.
is the diffusion coefficient.
Negative sign shows that diffusion is down the concentration gradient.
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
To make an introduction to a report of a simple design of a compressed air system, which uses the criterion of speed, and not that of pressure drop, to determine the diameter of the pipes, where the capacity of the compressor and the demands of the equipment are expressed in flow.
In an irrigation system, the following characteristics of the pipe network are available.• 100 meters of 4" PVC pipe, 3 gate valves• 500 meters of 3" PVC pipe, 4 gate valves• 200 meters of 2" H.G. pipe, 2 globe valves• 50 litres per second circulate in the pipes:Calculate:1. Total energy losses in meters.2. Leaks in pipes.3. Losses in accessories.4. Calculate the equivalent pipe of that system assuming only pipes without fittings.Solve the problem without artificial intelligence, solve by one of the experts
Liquid water enters the boiler at 60 bar. Steam exits the boiler at 60 bar, 540°C and undergoes a throttling process to 40 bar before entering the turbine. Steam expands adiabatically through the turbine to 5 bar, 240°C, and then undergoes a throttling process to 1 bar before entering the condenser. Kinetic and potential energy effects can be ignored.
Draw a Temperature-Entropy diagram and mark each of the states 2-5 on this diagram.
Determine the power generated by the turbine, in kJ per kg of steam flowing.
For the valves and the turbine, evaluate the rate of entropy production, each in kJ/K per kg of steam flowing.
Chapter 5 Solutions
The Science and Engineering of Materials (MindTap Course List)
Ch. 5 - What is the driving force for diffusion?Ch. 5 - Give three examples of materials processes that...Ch. 5 - In the carburization treatment of steels, what are...Ch. 5 - Prob. 5.4PCh. 5 - Prob. 5.5PCh. 5 - Prob. 5.6PCh. 5 - Prob. 5.7PCh. 5 - A certain mechanical component is heat treated...Ch. 5 - Prob. 5.9PCh. 5 - Prob. 5.10P
Ch. 5 - Prob. 5.11PCh. 5 - Prob. 5.12PCh. 5 - Prob. 5.13PCh. 5 - Prob. 5.14PCh. 5 - Prob. 5.15PCh. 5 - Prob. 5.16PCh. 5 - Compare the diffusion coefficients of carb on in...Ch. 5 - Prob. 5.18PCh. 5 - Activation energy is sometimes expressed as...Ch. 5 - Prob. 5.20PCh. 5 - The activation energy for the diffusion of copper...Ch. 5 - Prob. 5.22PCh. 5 - Prob. 5.23PCh. 5 - Prob. 5.24PCh. 5 - Prob. 5.25PCh. 5 - Write down Fick’s first law of diffusion. Clearly...Ch. 5 - Prob. 5.27PCh. 5 - Prob. 5.28PCh. 5 - Prob. 5.29PCh. 5 - Prob. 5.30PCh. 5 - A 1-mm-thick BCC iron foil is used to separate a...Ch. 5 - Prob. 5.32PCh. 5 - Prob. 5.33PCh. 5 - A 0.001 in. BCC iron foil is used to separate a...Ch. 5 - Prob. 5.35PCh. 5 - Prob. 5.36PCh. 5 - Prob. 5.37PCh. 5 - Prob. 5.38PCh. 5 - Prob. 5.39PCh. 5 - Prob. 5.40PCh. 5 - Prob. 5.41PCh. 5 - Prob. 5.42PCh. 5 - Prob. 5.43PCh. 5 - Prob. 5.44PCh. 5 - Prob. 5.45PCh. 5 - Prob. 5.46PCh. 5 - Prob. 5.47PCh. 5 - Prob. 5.48PCh. 5 - Pure zinc is to be diffused into copper by dipping...Ch. 5 - Nitriding is a process in which nitrogen is...Ch. 5 - Determine the carburizing time necessary to...Ch. 5 - Prob. 5.52PCh. 5 - Prob. 5.53PCh. 5 - Prob. 5.54PCh. 5 - Prob. 5.55PCh. 5 - Prob. 5.56PCh. 5 - Prob. 5.57PCh. 5 - Prob. 5.58PCh. 5 - Compare the rate at which oxygen ions diffuse in...Ch. 5 - Prob. 5.60PCh. 5 - Prob. 5.61PCh. 5 - Prob. 5.62PCh. 5 - Prob. 5.63PCh. 5 - Prob. 5.64PCh. 5 - Prob. 5.65PCh. 5 - A 0.80% C steel must operate at 950°C in an...Ch. 5 - Prob. 5.67PCh. 5 - Prob. 5.68PCh. 5 - Prob. 5.69PCh. 5 - Prob. 5.70PCh. 5 - Prob. 5.71PCh. 5 - Prob. 5.72PCh. 5 - Most metals and alloys can be processed using the...Ch. 5 - Prob. 5.74PCh. 5 - Prob. 5.75PCh. 5 - Prob. 5.76PCh. 5 - A ceramic part made from MgO is sintered...Ch. 5 - Prob. 5.78PCh. 5 - What are the advantages of using hot pressing and...Ch. 5 - Prob. 5.80PCh. 5 - Prob. 5.81DPCh. 5 - Design a spherical tank, with a will thickness of...Ch. 5 - Prob. 5.83DPCh. 5 - Prob. 5.84DPCh. 5 - Prob. 5.85DPCh. 5 - Prob. 5.86CPCh. 5 - Prob. 5.87CPCh. 5 - Prob. 5.88CPCh. 5 - Prob. 5.1KP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Find the componenets of reactions at pins of A, B and D please show the detailed process and instructions for learning draw out all diagrams please and thank youarrow_forwardA cast iron cylinder of 200 mm inner diameter and 12.5 mm thick is closely wound with a layer of 4 mm diameter steel wire under a tensile stress of 55 MN/m². Determine the stresses set up in the cylinder and steel wire if water under a pressure of 3 MN/m² is admitted in the cylinder. Take E= 100 GN/m², E = 200 GN/m² and Poisson's ratio = 0.25.arrow_forwardWhat is the effect of a clogged fuel injector?arrow_forward
- You are asked to design a unit to condense ammonia. The required condensation rate is 0.09kg/s. Saturated ammonia at 30 o C is passed over a vertical plate (10 cm high and 25 cm wide).The properties of ammonia at the saturation temperature of 30°C are hfg = 1144 ́10^3 J/kg andrho_v = 9.055 kg/m 3 . Use the properties of liquid ammonia at the film temperature of 20°C (Ts =10 o C):Pr = 1.463 rho_l= 610.2 kf/m^3 liquid viscosity= 1.519*10^-4 kg/ ms kinematic viscosity= 2.489*10^-7 m^2/s Cpl= 4745 J/kg C kl=0.4927 W/m C hfg*=hfg+0.68Cpl(Tsat-Given Ts) a) Instead of one plate you want to use small plates and install many of them. Calculate the requiredsurface temperature to achieve the desired condensation rate (0.09 kg/s) if you install 36vertical plates (with the same dimension as above: 10 cm high and 25 cm wide).arrow_forward11-19 designed in Problem The shaft shown in figure P11-4 was 10-19, for the data in the row(s) assigned from table PII-1, and the corresponding diameter of shaft found in Problem 10-19, design suitable bearings 5 E8 cycles at the load for at least State all assumptions. to support 1200rpm. (a) Using hydrodynamically lubricated bronze sleeve bearings with ON = 40, Lld = 0.8, and clearance ratio 0.0025. of a ← gear T gear Key figure PI-4 Given from the problem 10-19 we get d= 1.153 in from the table 11-1 we get a = 16 in b= 18in L= 20inarrow_forwardIn an irrigation system, the following characteristics of the pipe network are available.• 100 meters of 4" PVC pipe, 3 gate valves• 500 meters of 3" PVC pipe, 4 gate valves• 200 meters of 2" H.G. pipe, 2 globe valves• 50 litres per second circulate in the pipes:Calculate:1. Total energy losses in meters.2. Leaks in pipes.3. Losses in accessories.4. Calculate the equivalent pipe of that system assuming only pipes without fittings.Solve the problem without artificial intelligence, solve by one of the expertsarrow_forward
- In a series pipe, calculate the diameter 2 according to the following:• Ltotal: 325 m• L1: 52 m, D1: 3/4"• L2: 254 m, D2:?• L3: 19 m, D: 1-1/4".Indicate the nominal diameter. Solve without using artificial inteligence, solve by one of the expertsarrow_forwardWhat is the critical speed of the shaft in rad/s for one, two, and three elements?arrow_forward2. Express the following complex numbers in rectangular form. (a) z₁ = 2еjл/6 (b) Z2=-3e-jπ/4 (c) Z3 = √√√3e-j³/4 (d) z4 = − j³arrow_forward
- A prismatic beam is built into a structure. You can consider the boundary conditions at A and B to be fixed supports. The beam was originally designed to withstand a triangular distributed load, however, the loading condition has been revised and can be approximated by a cosine function as shown in the figure below. You have been tasked with analysing the structure. As the beam is prismatic, you can assume that the bending rigidity (El) is constant. wwo cos 2L x A B Figure 3: Built in beam with a varying distributed load In order to do this, you will: a. Solve the reaction forces and moments at point A and B. Hint: you may find it convenient to use the principal of superposition. (2%) b. Plot the shear force and bending moment diagrams and identify the maximum shear force and bending moment. (2%) c. Develop an expression for the vertical deflection. Clearly state your expression in terms of x. (1%)arrow_forwardQuestion 1: Beam Analysis Two beams (ABC and CD) are connected using a pin immediately to the left of Point C. The pin acts as a moment release, i.e. no moments are transferred through this pinned connection. Shear forces can be transferred through the pinned connection. Beam ABC has a pinned support at point A and a roller support at Point C. Beam CD has a roller support at Point D. A concentrated load, P, is applied to the mid span of beam CD, and acts at an angle as shown below. Two concentrated moments, MB and Mc act in the directions shown at Point B and Point C respectively. The magnitude of these moments is PL. Moment Release A B с ° MB = PL Mc= = PL -L/2- -L/2- → P D Figure 1: Two beam arrangement for question 1. To analyse this structure, you will: a) Construct the free body diagrams for the structure shown above. When constructing your FBD's you must make section cuts at point B and C. You can represent the structure as three separate beams. Following this, construct the…arrow_forwardA cantilevered rectangular prismatic beam has three loads applied. 10,000N in the positive x direction, 500N in the positive z direction and 750 in the negative y direction. You have been tasked with analysing the stresses at three points on the beam, a, b and c. 32mm 60mm 24mm 180mm 15mm 15mm 40mm 750N 16mm 500N x 10,000N Figure 2: Idealisation of the structure and the applied loading (right). Photograph of the new product (left). Picture sourced from amazon.com.au. To assess the design, you will: a) Determine state of stress at all points (a, b and c). These points are located on the exterior surface of the beam. Point a is located along the centreline of the beam, point b is 15mm from the centreline and point c is located on the edge of the beam. When calculating the stresses you must consider the stresses due to bending and transverse shear. Present your results in a table and ensure that your sign convention is clearly shown (and applied consistently!) (3%) b) You have identified…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Introduction to Diffusion in Solids; Author: Engineering and Design Solutions;https://www.youtube.com/watch?v=K_1QmKJvNjc;License: Standard youtube license