
To check
If 1+ cations of homonuclear diatomic molecules of the second-row elements always have shorter bond lengths than the corresponding neutral molecules.
Answer to Problem 5.105QA
Solution:
The cations of homonuclear diatomic molecules of the second row elements do not have shorter bond lengths than the corresponding neutral molecules.
Explanation of Solution
Bond order and bond length are inversely proportional to each other. When bond order increases the bond length decreases and vice versa. We will find out the bond order of neutral diatomic molecules and their 1+ cations. From that we will check if the bond length of the cation is shorter or not.
From the molecular orbital diagram we can find out number of bonding and antibonding electrons. The formula to calculate bond order is as below.
The molecular orbital diagram for the given molecule with the loss of two electrons forming the corresponding 1+ cation is as shown below. With the help of this diagram we will calculate bond order of each molecule and ion of the second row element.
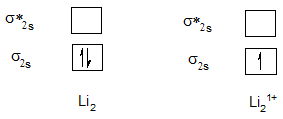
Bond order of Li2 and Li21+.
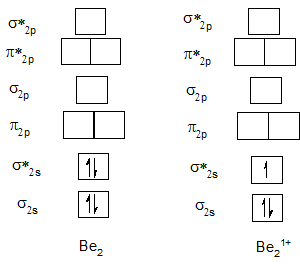
Bond order of Be2 and Be21+.
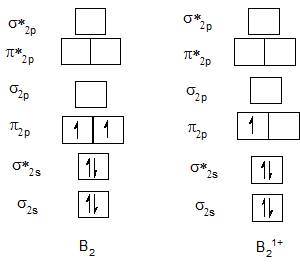
Bond order of B2 and B21+.
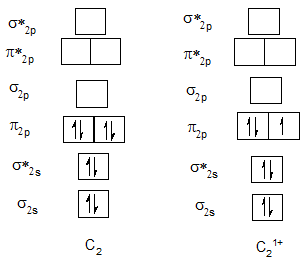
Bond order of C2 and C21+.
Bond order of N2 and N21+.
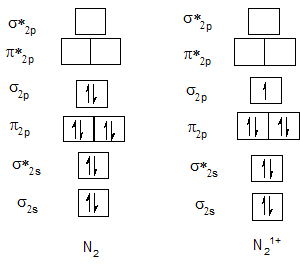
Bond order of O2 and O21+.
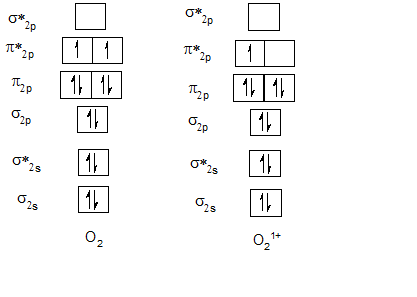
Bond order of F2 and F21+.
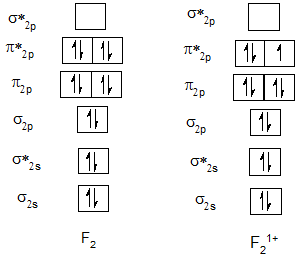
Bond order of Ne2 and Ne21+.
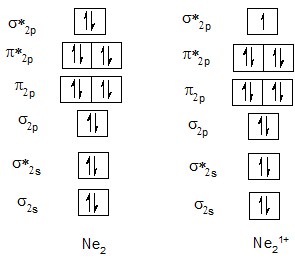
| Neutral diatomic molecule | Bond order of neutral molecule | Molecular cation | Bond order of cation |
| Li2 | 1 | Li21+ | 0.5 |
| Be2 | 0 | Be21+ | 0.5 |
| B2 | 1 | B21+ | 0.5 |
| C2 | 2 | C21+ | 1.5 |
| N2 | 3 | N21+ | 2.5 |
| O2 | 2 | O21+ | 2.5 |
| F2 | 1 | F21+ | 1.5 |
| He2 | 0 | He21+ | 0.5 |
From the bond order of neutral diatomic molecule and bond order of corresponding +1 cation we can say the bond order is not always increasing. Therefore, 1+ cations of homonuclear diatomic molecules of the second-row elements do not always have shorter bond lengths than the corresponding neutral molecules.
Conclusion:
Bond order is inversely proportional to bond length. From bond order of the neutral diatomic molecule and cations we cannot say the bond length is always shorter than neutral atom.
Want to see more full solutions like this?
Chapter 5 Solutions
Chemistry: An Atoms-Focused Approach
- 4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forward
- Draw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forward
- EFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardIf we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forwardIf we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forward
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardYou need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forwardDraw the formula for 3-isopropylcyclopentane-1-carbonyl chloride.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





