
Concept explainers
A diagram for an open-tube manometer is shown below.
If the flask is open to the atmosphere, the mercury levels are equal. For each of the following situations where a gas is contained in the flask, calculate the pressure in the flask in torr, atmospheres, and pascals.
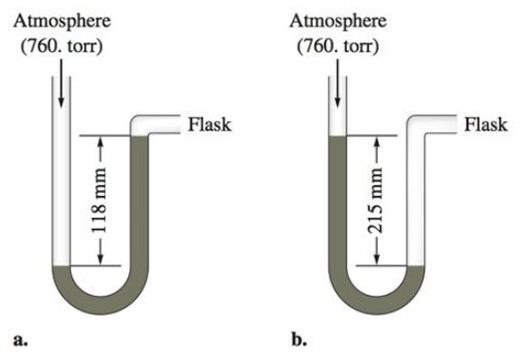
c. Calculate the pressures in the flask in parts a and b (in torr) if the atmospheric pressure is 635 torr.
(a)
Interpretation:
The pressure of the gases in given two situations of manometers (a) and (b) should be determined in units of torr, atm and pascals when the manometer shows a reading of 118mm and 215mm respectively. And also calculate the pressure of the gases in given two situations of manometers (a) and (b) If the atmospheric pressure is 635 torr.
Concept Introduction:
The manometer is a devise used measure the pressure of a gas. The pressure of gas is determined by the value of ‘h’ shown by the manometer. This ‘h’-value is added or subtracted with atmospheric pressure to determine the pressure of gas.
If the flask side mercury level is decreased after the filling of gas, then the ‘h’-value will be added to atmospheric pressure to get the pressure of gas.
If the flask side mercury level is increased after the filling of gas, then the ‘h’-value will be subtracted from the atmospheric pressure to get the pressure of gas.
The pressure equivalent of ‘h’ value is,
Pressure of a substance can be stated in various units. The units of pressure are interconvertible. The relations between units of pressure are,
- Since the unit mm Hg and the unit torr is used interchangeably.
- Conversion of 1 torr into atm is,
- The 1 mm Hg pressure in Pa unit is,
Answer to Problem 45E
The pressure of the given gas (figure-a) in units of torr, atm and pascal are,
642 torr, 0.8447 atm, 85593 Pa
Explanation of Solution
The given ‘h’ value for the gas in manometer is 118mm. The picture of manometer shows the flask side mercury level is increased after the filling of gas.
Hence the equation for finding the pressure of gas is,
That is,
=
=
The calculated pressure is 642 mm Hg; the mm Hg and torr units are used interchangeably,
Therefore,
The calculated pressure is 642mm Hg. So the pressure in atm unit is,
=
The calculated pressure is 642mm Hg. So the pressure in Pa unit is,
(b)
Interpretation:
The pressure of the gases in given two situations of manometers (a) and (b) should be determined in units of torr, atm and pascals when the manometer shows a reading of 118mm and 215mm respectively. And also calculate the pressure of the gases in given two situations of manometers (a) and (b) If the atmospheric pressure is 635 torr.
Concept Introduction:
The manometer is a devise used measure the pressure of a gas. The pressure of gas is determined by the value of ‘h’ shown by the manometer. This ‘h’-value is added or subtracted with atmospheric pressure to determine the pressure of gas.
If the flask side mercury level is decreased after the filling of gas, then the ‘h’-value will be added to atmospheric pressure to get the pressure of gas.
If the flask side mercury level is increased after the filling of gas, then the ‘h’-value will be subtracted from the atmospheric pressure to get the pressure of gas.
The pressure equivalent of ‘h’ value is,
Pressure of a substance can be stated in various units. The units of pressure are interconvertible. The relations between units of pressure are,
- Since the unit mm Hg and the unit torr is used interchangeably.
- Conversion of 1 torr into atm is,
- The 1 mm Hg pressure in Pa unit is,
Answer to Problem 45E
The pressure of the given gas (figure-b) in units of torr, atm and pascal are,
878 torr, 1.1552 atm, 117057 Pa
Explanation of Solution
The given ‘h’ value for the gas in manometer is 118mm. The picture of manometer shows the flask side mercury level is decreased after the filling of gas.
Hence the equation for finding the pressure of gas is,
That is,
=
=
The calculated pressure is 878 mm Hg; the mm Hg and torr units are used interchangeably,
Therefore,
The calculated pressure is 878mm Hg. So the pressure in atm unit is,
=
The calculated pressure is 878mm Hg. So the pressure in Pa unit is,
(c)
Interpretation:
The pressure of the gases in given two situations of manometers (a) and (b) should be determined in units of torr, atm and pascals when the manometer shows a reading of 118mm and 215mm respectively. And also calculate the pressure of the gases in given two situations of manometers (a) and (b) If the atmospheric pressure is 635 torr.
Concept Introduction:
The manometer is a devise used measure the pressure of a gas. The pressure of gas is determined by the value of ‘h’ shown by the manometer. This ‘h’-value is added or subtracted with atmospheric pressure to determine the pressure of gas.
If the flask side mercury level is decreased after the filling of gas, then the ‘h’-value will be added to atmospheric pressure to get the pressure of gas.
If the flask side mercury level is increased after the filling of gas, then the ‘h’-value will be subtracted from the atmospheric pressure to get the pressure of gas.
The pressure equivalent of ‘h’ value is,
Pressure of a substance can be stated in various units. The units of pressure are interconvertible. The relations between units of pressure are,
- Since the unit mm Hg and the unit torr is used interchangeably.
- Conversion of 1 torr into atm is,
- The 1 mm Hg pressure in Pa unit is,
Answer to Problem 45E
The pressure of the given gas (figure-a) in units of torr, atm and pascal when the atmospheric pressure is 635 torr are,
517 torr, 0.8141 atm, 82496 Pa
The pressure of the given gas (figure-b) in units of torr, atm and pascal when the atmospheric pressure is 635 torr are,
753 torr, 1.1858 atm, 120154 Pa
Explanation of Solution
The pressure of the gas in given situation of manometer (a) in units of torr, atm and pascals:
The given ‘h’ value for the gas in manometer (a) is 118mm. The picture of manometer shows the flask side mercury level is increased after the filling of gas.
Hence the equation for finding the pressure of gas is,
That is,
=
=
The calculated pressure is 517 mm Hg; the mm Hg and torr units are used interchangeably,
Therefore,
The calculated pressure is 517mm Hg. So the pressure in atm unit is,
=
The calculated pressure is 517mm Hg. So the pressure in Pa unit is,
The pressure of the gas in given situation of manometer (b) in units of torr, atm and pascals:
The given ‘h’ value for the gas in manometer is 118mm. The picture of manometer shows the flask side mercury level is decreased after the filling of gas.
Hence the equation for finding the pressure of gas is,
That is,
=
=
The calculated pressure is 753 mm Hg; the mm Hg and torr units are used interchangeably,
Therefore,
The calculated pressure is
=
The calculated pressure is
Want to see more full solutions like this?
Chapter 5 Solutions
Bundle: Chemistry, Loose-leaf Version, 10th + Enhanced Webassign Printed Access Card For Chemistry, Multi-term Courses
- What is the IUPAC name of the following compound? CH₂CH₂ H CI H₂CH₂C H CH₂ Selected Answer: O (35,4R)-4 chloro-3-ethylpentane Correctarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electrons-pushing arrows for the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. I I I H Select to Add Arrows HCI, CH3CH2OHarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and the follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the intermediates and product of the following reaction or mechanistic step(s).arrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and the product in this reaction or mechanistic step(s).arrow_forward
- Look at the following pairs of structures carefully to identify them as representing a) completely different compounds, b) compounds that are structural isomers of each other, c) compounds that are geometric isomers of each other, d) conformers of the same compound (part of structure rotated around a single bond) or e) the same structure.arrow_forwardGiven 10.0 g of NaOH, what volume of a 0.100 M solution of H2SO4 would be required to exactly react all the NaOH?arrow_forward3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forward
- 3.50 g of Li are combined with 3.50 g of N2. What is the maximum mass of Li3N that can be produced? 6 Li + N2 ---> 2 Li3Narrow_forwardConcentration Trial1 Concentration of iodide solution (mA) 255.8 Concentration of thiosulfate solution (mM) 47.0 Concentration of hydrogen peroxide solution (mM) 110.1 Temperature of iodide solution ('C) 25.0 Volume of iodide solution (1) used (mL) 10.0 Volume of thiosulfate solution (5:03) used (mL) Volume of DI water used (mL) Volume of hydrogen peroxide solution (H₂O₂) used (mL) 1.0 2.5 7.5 Time (s) 16.9 Dark blue Observations Initial concentration of iodide in reaction (mA) Initial concentration of thiosulfate in reaction (mA) Initial concentration of hydrogen peroxide in reaction (mA) Initial Rate (mA's)arrow_forwardDraw the condensed or line-angle structure for an alkene with the formula C5H10. Note: Avoid selecting cis-/trans- isomers in this exercise. Draw two additional condensed or line-angle structures for alkenes with the formula C5H10. Record the name of the isomers in Data Table 1. Repeat steps for 2 cyclic isomers of C5H10arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





