
ORGANIC CHEMISTRY-ACCESS
6th Edition
ISBN: 9781260475586
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 43P
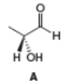 Determine if each compound is identical to or an enantiomer of A.
Determine if each compound is identical to or an enantiomer of A.
a. 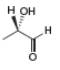 b.
b. 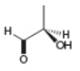 c.
c. 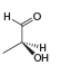
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please help me figure out what the slope is and how to calculate the half life Using the data provided.
Curved arrows are used to illustrate the flow of electrons. Follow
the curved arrows and draw the structure of the missing
reactants, intermediates, or products in the following mechanism.
Include all lone pairs. Ignore stereochemistry. Ignore inorganic
byproducts.
H
Br2 (1 equiv)
H-
Select to Draw
Starting Alkene
Draw Major
Product
I
I
H2O
四:
⑦..
Q
Draw Major
Charged
Intermediate
I
NH (aq)+CNO (aq) → CO(NH2)2(s)
Experiment
[NH4] (M) [CNO] (M) Initial rate (M/s)
1
0.014
0.02
0.002
23
0.028
0.02
0.008
0.014
0.01
0.001
Calculate the rate contant for this reaction using the data provided in the table.
Chapter 5 Solutions
ORGANIC CHEMISTRY-ACCESS
Ch. 5.1 - Prob. 1PCh. 5.2 - Prob. 2PCh. 5.3 - Draw the mirror image of each compound. Label each...Ch. 5.3 - Prob. 4PCh. 5.3 - A molecule is achiral if it has a plane of...Ch. 5.6 - Prob. 17PCh. 5.6 - Prob. 18PCh. 5.7 - Prob. 19PCh. 5.7 - Prob. 20PCh. 5.7 - Problem 5.18 Compounds E and F are two isomers of...
Ch. 5.8 - Prob. 22PCh. 5.8 - Prob. 23PCh. 5.8 - Prob. 24PCh. 5.9 - Prob. 25PCh. 5.9 - Prob. 26PCh. 5.9 - Prob. 27PCh. 5.10 - Which of the following cyclic molecules are meso...Ch. 5.10 - Prob. 29PCh. 5.11 - Prob. 30PCh. 5.12 - Problem 5.28 The amino acid has the physical...Ch. 5.12 - Prob. 32PCh. 5.12 - Prob. 33PCh. 5.12 - Prob. 34PCh. 5.12 - Prob. 35PCh. 5 - Prob. 39PCh. 5 - Prob. 40PCh. 5 - Prob. 41PCh. 5 - Prob. 42PCh. 5 - 5.40 Determine if each compound is identical to or...Ch. 5 - Prob. 44PCh. 5 - Prob. 45PCh. 5 - Prob. 46PCh. 5 - Prob. 47PCh. 5 - Prob. 48PCh. 5 - Prob. 52PCh. 5 - Prob. 56PCh. 5 - Prob. 61PCh. 5 - Prob. 67PCh. 5 - Prob. 68PCh. 5 - Prob. 69PCh. 5 -
5.67 Artemisinin and mefloquine are widely used...Ch. 5 - 5.68 Saquinavir (trade name Invirase) is a...Ch. 5 - Prob. 72P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Single penny tossed 20 times and counting heads and tails: Probability (prediction): _______/20 heads ________/...
Laboratory Manual For Human Anatomy & Physiology
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
More than one choice may apply. Using the terms listed below, fill in the blank with the proper term. anterior ...
Essentials of Human Anatomy & Physiology (12th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2CIO2 + 20H-1 CIO31 + CIO2 + H2O Experiment [CIO2], M [OH-1], M 1 0.0500 0.100 23 2 0.100 0.100 3 0.100 0.0500 Initial Rate, M/s 0.0575 0.230 0.115 ... Given this date, calculate the overall order of this reaction.arrow_forward2 3 .(be)_[Ɔ+(be)_OI ← (b²)_IƆO+ (be)_I Experiment [1-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 0.000069 4 0.0025 0.0025 0.000140 Calculate the rate constant of this reaction using the table data.arrow_forward1 2 3 4 I(aq) +OCl(aq) → IO¯¯(aq) + Cl¯(aq) Experiment [I-] M 0.005 [OCI-] 0.005 Initial Rate M/min 0.000275 0.0025 0.005 0.000138 0.0025 0.0025 Calculate the overall order of this reaction using the table data. 0.0025 0.000069 0.0025 0.000140arrow_forward
- H2O2(aq) +3 I¯(aq) +2 H+(aq) → 13(aq) +2 H₂O(l)· ••• Experiment [H2 O2]o (M) [I]o (M) [H+]。 (M) Initial rate (M/s) 1 0.15 0.15 0.05 0.00012 234 0.15 0.3 0.05 0.00024 0.3 0.15 0.05 0.00024 0.15 0.15 0.1 0.00048 Calculate the overall order of this reaction using the table data.arrow_forwardThe U. S. Environmental Protection Agency (EPA) sets limits on healthful levels of air pollutants. The maximum level that the EPA considers safe for lead air pollution is 1.5 μg/m³ Part A If your lungs were filled with air containing this level of lead, how many lead atoms would be in your lungs? (Assume a total lung volume of 5.40 L.) ΜΕ ΑΣΦ = 2.35 1013 ? atoms ! Check your rounding. Your final answer should be rounded to 2 significant figures in the last step. No credit lost. Try again.arrow_forwardY= - 0.039 (14.01) + 0.7949arrow_forward
- Suppose 1.76 g of magnesium acetate (Mg (CH3CO2)2) are dissolved in 140. mL of water. Find the composition of the resulting electrolyte solution. In particular, list the chemical symbols (including any charge) of each dissolved ion in the table below. List only one ion per row. mEq Then, calculate the concentration of each ion in dwrite the concentration in the second column of each row. Be sure you round your answers to the L correct number of significant digits. ion Add Row mEq L x 5arrow_forwardA pdf file of your hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). (A) do the work on a tablet and save as a pdf., it is expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise and every bond that is formed and broken in the process of the reaction, and is expected to see all relevant lone pair electrons and curved arrows.arrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY