
Concept explainers
(a)
Interpretation: The stereogenic centers in the ball-and-stick model of ezetimibe are to be located.
Concept introduction: A carbon atom that has four nonequivalent atoms or groups attached to it is known as chiral carbon atom. Chiral carbon centers are also called as asymmetric or stereogenic centers.
Answer to Problem 39P
The stereogenic centers in ezetimibe are,
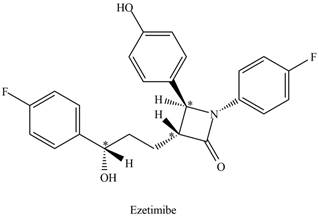
Explanation of Solution
The given ball-and-stick model of ezetimibe is shown below.
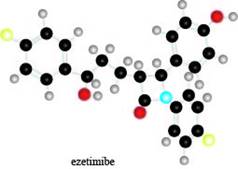
Figure 1
In the ball-and-stick model of ezetimibe, yellow balls represent halogen atom as they contain one covalent bond. Black balls are bonded to each other through four covalent bonds, thus; they represent carbon atom. Red balls represent oxygen atom as they contain two covalent bonds and blue balls represent nitrogen atom because they contain three covalent bonds.
The stereogenic center is one that has four different groups attached to carbon atom tetrahedrally. The stereogenic centers in the given molecule are located by omitting all
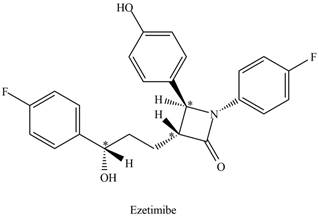
Figure 2
There are three stereogenic centers present in ezetimibe.
There are three stereogenic centers present in ezetimibe.
(b)
Interpretation: Each stereogenic center in ezetimibe are to be labeled as
Concept introduction: A carbon atom that has four nonequivalent atoms or groups attached to it is known as chiral carbon atom. Chiral carbon centers are also called as asymmetric or stereogenic centers.
The naming of chiral center and geometric isomers are based on Cahn-Ingold-Prelog priority rules. If the priority assigned to each group attached to the chirality center in a molecule is in a clockwise direction, then it is the R-stereoisomer, and if this is counter-clockwise, then it is the S-stereoisomer. R and S-stereoisomer are mirror images of each other.
Answer to Problem 39P
The
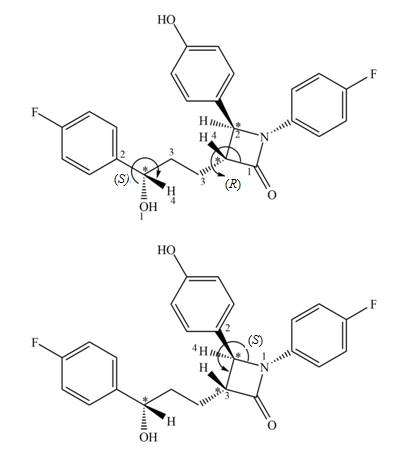
Explanation of Solution
The
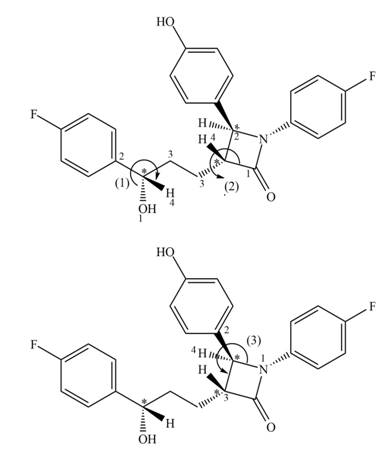
Figure 3
The stereogenic center (1) has
In ezetimibe, one of the stereogenic centers has
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY
- Name the following carbohydrates give both the systematic and common names. Don't forget to identify the Isomer.arrow_forwardWhat is the product of the reaction of XeF4 with H2O? Group of answer choices H2XeF2 H2XeF4 XeO3 H2XeOarrow_forwardWhile noble gas exerts the strongest London (dispersion) forces on neighboring atoms? Group of answer choices Xe Ar Kr Nearrow_forward
- Which of the following elements is corrosive to your skin due to that element breaking down C=C bonds? Group of answer choices fluorine iodine bromine chlorinearrow_forwardWhat the best source of sulfide to use on a small scale in the lab? Group of answer choices thiourea H2S NaHS Na2Sarrow_forwardWhich of the following statements about sulfur is FALSE? Group of answer choices H2S is the product of an oxygen-depleted ecosystem. In the acid mine drainage reaction, FeS2 is a product. One allotrope of sulfur has the formula S20. In the environment, bacterial oxidation can convert S2− to elemental S or SO42−.arrow_forward
- Of the following choices, which is the best reason that most materials DON'T spontaneously combust even though our atmosphere is about 21% oxygen? Group of answer choices The reduction of O2 in the gas phase (O2 + e− → O2−) is spontaneous. The reduction of O2 in acid solution (O2 + H+ + e− → HO2(aq)) is spontaneous. O2 is not a reactant in combustion. The O2 bond dissociation energy is 494 kJ/mol, leading to a high activation energy for combustion.arrow_forwardplease answer in the scope of the SCH4U course, I am having a hard time understanding, may you show all steps please and thank you! can you also put the final answers in the table so its understandablearrow_forwardPlan the synthesis of the following compound using the starting material provided and any other reagents needed as long as carbon based reagents have 3 carbons or less. Either the retrosynthesis or the forward synthesis (mechanisms are not required but will be graded if provided) will be accepted if all necessary reagents and intermediates are shown (solvents and temperature requirements are not needed unless specifically involved in the reaction, i.e. DMSO in the Swem oxidation or heat in the KMnO4 oxidation). There may be more than one correct answer, and chemically correct steps will be accepted. Extra points will be given if correct names are provided. The points earned here will be applied to your lowest exam score! H Harrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


