
Write structural formulas for all the constitutionally isomeric alcohols of molecular
formula
Interpretation:
The structural formulas for the constitutionally isomeric alcohols of molecular formula
Concept Introduction:
Isomers that have the same molecular formula but differ in the way in which different atoms are connected are known as constitutional isomers.
The number of atoms of each element present in the compound, is known as the molecular formula.
When the functional group
Answer to Problem 22P
Solution:
There are 8 constitutionally isomeric alcohols of molecular formula
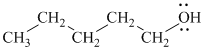
Substitutive name:
Functional class name: pentyl alcohol
It is a primary alcohol.
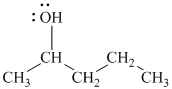
Substitutive name:
Functional class name:
It is a secondary alcohol.
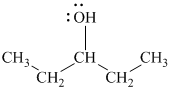
Substitutive name:
Functional class name:
It is a secondary alcohol.
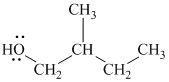
Substitutive name:
Functional class name:
It is a primary alcohol.
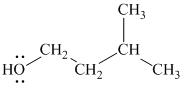
Substitutive name:
Functional class name:
It is a primary alcohol.
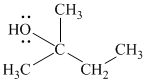
Substitutive name:
Functional class name:
It is a tertiary alcohol.
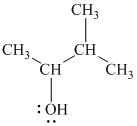
Substitutive name:
Functional class name:
It is a secondary alcohol.
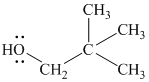
Substitutive name:
Functional class name:
It is a primary alcohol.
Explanation of Solution
In all, there are
They have the same molecular formula but different connectivity of atoms.
The structural formulas of these alcohols can be written as follows.
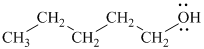
The substitutive name of this alcohol is
The functional class name of this alcohol is pentyl alcohol.
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
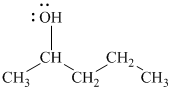
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a secondary carbon atom. Hence, it is a secondary alcohol.
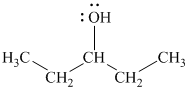
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a secondary carbon atom. Hence, it is a secondary alcohol.
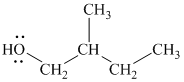
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
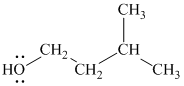
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
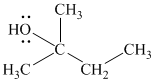
The substitutive name of this alcohol is 2-methyl-2-butanol.
The functional class name of this alcohol is 1,1-dimethylpropanol.
The hydroxyl group is attached to a tertiary carbon atom. Hence, it is a tertiary alcohol.
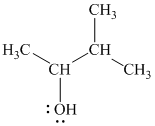
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a secondary carbon atom. Hence, it is a secondary alcohol.
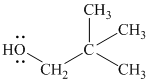
The substitutive name of this alcohol is
The functional class name of this alcohol is
The hydroxyl group is attached to a primary carbon atom. Hence, it is a primary alcohol.
The structural formulas for the constitutionally isomeric alcohols of molecular formula
Want to see more full solutions like this?
Chapter 5 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- Briefly explain chemical potential.arrow_forwardReason whether it is possible to determine changes in the Galvani potential difference at the metal-solution interface.arrow_forwardObtain the standard potential at 25°C of the Cu* I Cu | Pt electrode from the standard potentials E° Cu²+/Cu = 0.341 V and E Cu²+ /Cu+ = 0.153 V.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning





