
Foundations of College Chemistry, Binder Ready Version
15th Edition
ISBN: 9781119083900
Author: Morris Hein, Susan Arena, Cary Willard
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 5, Problem 20PE
(a)
Interpretation Introduction
Interpretation:
The isotopic notation symbol for
Concept Introduction:
Mass number is the sum of the protons and neutrons in the nucleus.
For an element
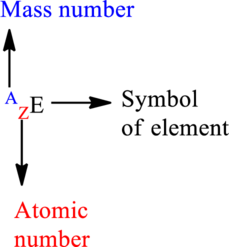
The letter A represents mass number.
The letter Z represents atomic number.
(b)
Interpretation Introduction
Interpretation:
The isotopic notation symbol for
Concept Introduction:
Refer to part (a).
(c)
Interpretation Introduction
Interpretation:
The isotopic notation symbol for
Concept Introduction:
Refer to part (a).
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Synthesis of 2-metilbenzimidazol from 1,2-diaminobenceno y propanona.
Predict the product of the following reaction.
1st attempt
HI
1
product
50300
Jul See Periodic Table
See Hint
P
Br
石尚
I
Indicate the substitutes in one place, if they are a diazonio room.
Chapter 5 Solutions
Foundations of College Chemistry, Binder Ready Version
Ch. 5.1 - Prob. 5.1PCh. 5.2 - Prob. 5.2PCh. 5.3 - Prob. 5.3PCh. 5.4 - Prob. 5.4PCh. 5.5 - Prob. 5.5PCh. 5.5 - Prob. 5.6PCh. 5.6 - Prob. 5.7PCh. 5 - Prob. 1RQCh. 5 - Prob. 2RQCh. 5 - Prob. 3RQ
Ch. 5 - Prob. 4RQCh. 5 - Prob. 5RQCh. 5 - Prob. 6RQCh. 5 - Prob. 7RQCh. 5 - Prob. 8RQCh. 5 - Prob. 9RQCh. 5 - Prob. 10RQCh. 5 - Prob. 11RQCh. 5 - Prob. 12RQCh. 5 - Prob. 1PECh. 5 - Prob. 2PECh. 5 - Prob. 3PECh. 5 - Prob. 4PECh. 5 - Prob. 5PECh. 5 - Prob. 6PECh. 5 - Prob. 7PECh. 5 - Prob. 8PECh. 5 - Prob. 9PECh. 5 - Prob. 10PECh. 5 - Prob. 11PECh. 5 - Prob. 12PECh. 5 - Prob. 13PECh. 5 - Prob. 14PECh. 5 - Prob. 15PECh. 5 - Prob. 16PECh. 5 - Prob. 17PECh. 5 - Prob. 18PECh. 5 - Prob. 19PECh. 5 - Prob. 20PECh. 5 - Prob. 21PECh. 5 - Prob. 22PECh. 5 - Prob. 23PECh. 5 - Prob. 24PECh. 5 - Prob. 25PECh. 5 - Prob. 26PECh. 5 - Prob. 27PECh. 5 - Prob. 28PECh. 5 - Prob. 29PECh. 5 - Prob. 30PECh. 5 - Prob. 31PECh. 5 - Prob. 32PECh. 5 - Prob. 33PECh. 5 - Prob. 34PECh. 5 - Prob. 35AECh. 5 - Prob. 36AECh. 5 - Prob. 37AECh. 5 - Prob. 38AECh. 5 - Prob. 39AECh. 5 - Prob. 42AECh. 5 - Prob. 43AECh. 5 - Prob. 45AECh. 5 - Prob. 46AECh. 5 - Prob. 47AECh. 5 - Prob. 48AECh. 5 - Prob. 49AECh. 5 - Prob. 50AECh. 5 - Prob. 51AECh. 5 - Prob. 53AECh. 5 - Prob. 54AECh. 5 - Prob. 55AECh. 5 - Prob. 56AECh. 5 - Prob. 60CE
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the product formed in each reaction. If the product exhibits tautomerism, draw the tautomeric structure. a) о + CH3-NH-NH2 CO2C2H5 b) + CoH5-NH-NH2 OC2H5arrow_forwardIndicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forwardSynthesis of 1-metilbenzotriazole from 1,2-diaminobenceno.arrow_forward
- Synthesis of 1-metilbenzotriazole.arrow_forwardIndicate the formula of the compound, that is the result of the N- alquilación (nucleofílic substitution), in which an additional lateral chain was formed (NH-CH2-COOMe). F3C. CF3 NH NH2 Br о OMe K2CO3, DABCO, DMFarrow_forwardIdentify the mechanism through which the following reaction will proceed and draw the major product. Part 1 of 2 Br KOH EtOH Through which mechanism will the reaction proceed? Select the single best answer. E1 E2 neither Part: 1/2 Part 2 of 2 Draw the major product formed as a result of the reaction. Click and drag to start drawing a structure. Xarrow_forward
- What is single-point calibration? Provide an example.arrow_forwardDraw the major product formed via an E1 pathway.arrow_forwardPart 9 of 9 Consider the products for the reaction. Identify the major and minor products. HO Cl The E stereoisomer is the major product and the Z stereoisomer is the minor product ▼ S major product minor productarrow_forward
- Consider the reactants below. Answer the following questions about the reaction mechanism and products. HO Clarrow_forwardjulietteyep@gmail.com X YSCU Grades for Juliette L Turner: Orc X 199 A ALEKS - Juliette Turner - Modul X A ALEKS - Juliette Turner - Modul x G butane newman projection - Gox + www-awa.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBxzaplnN4HsoQggFsejpgqKoyrQrB2dKVAN-BcZvcye0LYa6eXZ8d4vVr8Nc1GZqko5mtw-d1MkNcNzzwZsLf2Tu9_V817y?10Bw7QYjlb il Scribbr citation APA SCU email Student Portal | Main Ryker-Learning WCU-PHARM D MySCU YSCU Canvas- SCU Module 4: Homework (Ch 9-10) Question 28 of 30 (1 point) | Question Attempt: 1 of Unlimited H₂SO heat OH The mechanism of this reaction involves two carbocation intermediates, A and B. Part 1 of 2 KHSO 4 rearrangement A heat B H₂O 2 OH Draw the structure of A. Check Search #t m Save For Later Juliet Submit Assignm 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessarrow_forwardThe electrons flow from the electron-rich atoms of the nucleophile to the electrons poor atoms of the alkyl halide. Identify the electron rich in the nucleophile. Enter the element symbol only, do not include any changes.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning