
Concept explainers
Biofuels
A lot of energy is locked up in the
Corn, soy, sugarcane, and other food crops are rich in oils, starches, and sugars that can be easily converted to biofuels. The starch in corn kernels, for example, can be enzymatically broken down to glucose, which is fermented to ethanol by bacteria or yeast. However, growing food crops for biofuel production typically requires a lot of energy (in the form of fossil fuels) and it damages the environment. Making biofuels from other plant matter such as weeds or agricultural waste requires additional steps, because these materials contain a higher proportion of cellulose. Breaking down this tough carbohydrate to its glucose monomers adds cost to the biofuel product.
In 2006, David Tilman and his colleagues published the results of a 10-year study comparing the net energy output of various biofuels. The researchers made biofuel from a mixture of native perennial grasses grown without irrigation, fertilizer, pesticides, or herbicides, in sandy soil that was so depleted by intensive agriculture that it had been abandoned. The energy content of this biofuel and the energy it took to produce it were measured and compared with that of biofuels made from food crops (Figure 5.16).
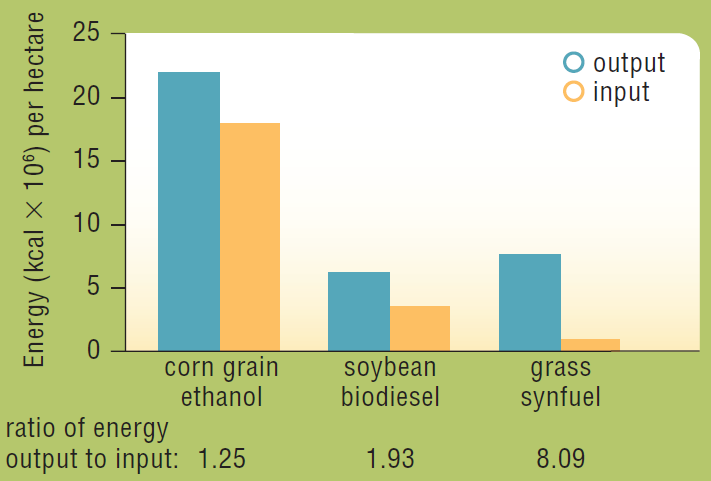
About how much energy did ethanol produced from one hectare of corn yield? How much energy did it take to grow and produce that ethanol?
To determine: How much energy did the ethanol produced from one hectare of corn yield and how much energy did it took to grow the corn.
Concept introduction:The plants and other organic material other than fossils can also be used as a source of energy. The oils, gases, and alcohols made from these materials are called biofuels. Corn and other food crops are rich in oils, starches, and sugars that can be easily converted into biofuels.
Explanation of Solution
The researcher D and his colleagues studied for 10 years and compared the net energy output of various biofuels. The researcher grew a mixture of native perennial grasses, corn, and soy. The grasses grew without irrigation, fertilizer, and pesticides in sandy soil. The usable energy in biofuel (grasses, corn, and soy) is measured along with the energy it took to grow.
Refer to Fig. 6.1 “Energy inputs and outputs of biofuels made from three different crops” in the question. The graph plot shows the energy per hectare versus the ratio of energy output to input of the three different biofuels.
The graph shows the ethanol obtained from one hectare of corn produced
The ethanol from one hectare of corn produced approximately 23 × 106 kcal. It took approximately 18 × 106 kcal energy to grow the corn that was used to make the ethanol.
Want to see more full solutions like this?
Chapter 5 Solutions
EBK BIOLOGY TODAY AND TOMORROW WITH PHY
- How is a protein destined for the Endoplasmic Reticulum (ER), imported into the ER? Be concise.arrow_forwardFind out about the organisations and the movements aimed at the conservation of our natural resources. Eg Chipko movement and Greenpeace. Make a project report on such an organisation.arrow_forwardWhat are biofertilizers and mention the significancearrow_forward
- PCBs and River Otters: Otters in Washington State’s Green-Duwamish River have high levels of polychlorinated biphenyls (PCBs) in their livers. PCBs can bind to the estrogen receptors in animals and disrupt the endocrine system of these otters. The PCBs seem to increase the estrogen to androgen ratio, skewing the ratio toward too much estrogen. How would increased estrogen affect the river otter population? Based on your reading of the materials in this unit, what factors can affect fertility in humans? Explain how each of the factors affecting human fertility that you described can disrupt the human endocrine system to affect reproduction.arrow_forwardOther than oil and alcohol, are there other liquids you could compare to water (that are liquid at room temperature)? How is water unique compared to these other liquids? What follow-up experiment would you like to do, and how would you relate it to your life?arrow_forwardSelection of Traits What adaptations do scavengers have for locating and feeding on prey? What adaptations do predators have for capturing and consuming prey?arrow_forward
- Competition Between Species What natural processes limit populations from growing too large? What are some resources organisms can compete over in their natural habitat?arrow_forwardSpecies Interactions Explain how predators, prey and scavengers interact. Explain whether predators and scavengers are necessary or beneficial for an ecosystem.arrow_forwardmagine that you are conducting research on fruit type and seed dispersal. You submitted a paper to a peer-reviewed journal that addresses the factors that impact fruit type and seed dispersal mechanisms in plants of Central America. The editor of the journal communicates that your paper may be published if you make ‘minor revisions’ to the document. Describe two characteristics that you would expect in seeds that are dispersed by the wind. Contrast this with what you would expect for seeds that are gathered, buried or eaten by animals, and explain why they are different. (Editor’s note: Providing this information in your discussion will help readers to consider the significance of the research).arrow_forward
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
 Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning





