
Chemistry (OER)
19th Edition
ISBN: 9781947172623
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 5, Problem 11E
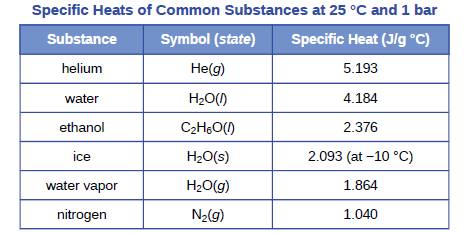
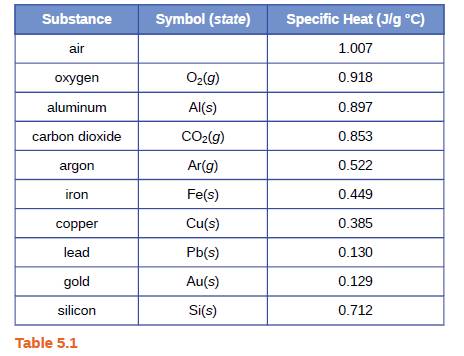
A piece of unknown solid substance weighs 437.2 g, and requires 8460 J to increase its temperature from 19.3 °C to 68.9 °C.
(a) What is the specific heat of the substance?
(b) If it is one of the substances found in Table 5.1, what is its likely identity?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
C.
Ν
H
a.
H3C.
N
H3C
CH3
HCN
ол
2.
восцапан
(46:00)
Curtius rearrangment
1. NaN3, heat
-OH
Chapter 5 Solutions
Chemistry (OER)
Ch. 5 - A burning match and a bonfire may have the same...Ch. 5 - Prepare a table identifying several energy...Ch. 5 - Explain the difference between heat capacity and...Ch. 5 - Calculate the heat capacity, in joules and in...Ch. 5 - Calculate the heat capacity, in joules and in...Ch. 5 - How much heat, in joules and in calories, must be...Ch. 5 - How much heat, in joules and in calories, is...Ch. 5 - How much would the temperature of 275 g of water...Ch. 5 - If 14.5 kJ of heat were added to 485 g of liquid...Ch. 5 - A piece of unknown substance weighs 44.7 g and...
Ch. 5 - A piece of unknown solid substance weighs 437.2 g,...Ch. 5 - An aluminum kettle weighs 1.05 kg. (a) What is the...Ch. 5 - Most people find waterbeds uncomfortable unless...Ch. 5 - A 500-mL bottle of water at room temperature and a...Ch. 5 - Would the amount of heat measured for the reaction...Ch. 5 - Would the amount of heat absorbed by the...Ch. 5 - Would the amount of heat absorbed by the...Ch. 5 - How many milliliters of water at 23 C with a...Ch. 5 - How much will the temperature of a cup (180 g) of...Ch. 5 - A 45-g aluminum spoon (specific heat 0.88 J/g C)...Ch. 5 - The temperature of the cooling water as it leaves...Ch. 5 - A 70.0-g piece of metal at 80.0 °C is placed in...Ch. 5 - If a reaction produces 1.506 kJ of heat, which is...Ch. 5 - A 0.500-g sample of KCl is added to 50.0 g of...Ch. 5 - Dissolving 3.0 g of CaCl2(s) in 150.0 g of water...Ch. 5 - When 50.0 g of 0.200 M NaCl(aq) at 24.1 C is added...Ch. 5 - The addition of 3.15 g of Ba(OH)28H2O to a...Ch. 5 - The reaction of 50 mL of acid and 50 mL of base...Ch. 5 - If the 3.21 g of NH4NO3 in Example 5.6 were...Ch. 5 - When 1.0 g of fructose, C6H12O6(s), a sugar...Ch. 5 - When a 0.740-g sample of trinitrotoluene (TNT),...Ch. 5 - One method of generating electricity is by burning...Ch. 5 - The amount of fat recommended for someone with a...Ch. 5 - A teaspoon of the carbohydrate sucrose (common...Ch. 5 - What is the maximum mass of carbohydrate in a 6-oz...Ch. 5 - A pint of premium ice cream can contain 1100...Ch. 5 - A serving of a breakfast cereal contains 3 g of...Ch. 5 - Which is the least expensive source of energy in...Ch. 5 - Explain how the heat measured in Example 5.5...Ch. 5 - Using the data in the check your learning section...Ch. 5 - Calculate the enthalpy of solution ( H for the...Ch. 5 - Calculate H for the reaction described by the...Ch. 5 - Calculate the enthalpy of solution ( H for the...Ch. 5 - Although the gas used in an oxyacetylene torch...Ch. 5 - How much heat is produced by burning 4.00 moles of...Ch. 5 - How much heat is produced by combustion of 125 g...Ch. 5 - How many moles of isooctane must be burned to...Ch. 5 - What mass of carbon monoxide must be burned to...Ch. 5 - When 2.50 g of methane burns in oxygen, 125 kJ of...Ch. 5 - How much heat is produced when loo mL of 0.250 M...Ch. 5 - A sample of 0.562 g of carbon is burned in oxygen...Ch. 5 - Before the introduction of chlorofluorocarbons,...Ch. 5 - Homes may be heated by pumping hot water through...Ch. 5 - Which of the enthalpies of combustion in Table 5.2...Ch. 5 - Does the standard enthalpy of formation of H2O(g)...Ch. 5 - Joseph Priestly prepared oxygen in 1774 by heating...Ch. 5 - How many kilojoules of heat will be released when...Ch. 5 - How many kilojoules of heat will be released when...Ch. 5 - The following sequence of reactions occurs in the...Ch. 5 - Both graphite and diamond burn....Ch. 5 - From the molar heats of formation in Appendix G,...Ch. 5 - Which produces more heat?...Ch. 5 - Calculate H298 for the process...Ch. 5 - Calculate H298 for the process...Ch. 5 - Calculate H for the process Hg2Cl2(s)2Hg(l)+Cl2(g)...Ch. 5 - Calculate H298 for the process...Ch. 5 - Calculate the standard molar enthalpy of formation...Ch. 5 - Using the data in Appendix G, calculate the...Ch. 5 - Using the data in Appendix G, calculate the...Ch. 5 - The following reactions can be used to prepare...Ch. 5 - The decomposition of hydrogen peroxide, H2O2, has...Ch. 5 - Calculate the enthalpy of combustion of propane,...Ch. 5 - Calculate the enthalpy of combustion of butane,...Ch. 5 - Both propane and butane are used as gaseous fuels....Ch. 5 - The white pigment TiO2 is prepared by the reaction...Ch. 5 - Water gas, a mixture of H2 and CO, is an important...Ch. 5 - In the early days of automobiles, illumination at...Ch. 5 - From the data in Table 5.2, determine which of the...Ch. 5 - The enthalpy of combustion of hard coal averages...Ch. 5 - Ethanol, C2H5OH, is used as a fuel for motor...Ch. 5 - Among the substances that react with oxygen and...Ch. 5 - How much heat is produced when 1.25 g of chromium...Ch. 5 - Ethylene, C2H2, a byproduct from the fractional...Ch. 5 - The oxidation of the sugar glucose, C6H12O6, is...Ch. 5 - Propane, C3H8, is a hydrocarbon that is commonly...Ch. 5 - During a recent winter month in Sheboygan,...
Additional Science Textbook Solutions
Find more solutions based on key concepts
True or false? Some trails are considered vestigial because they existed long ago.
Biological Science (6th Edition)
17. Anthropologists are interested in locating areas in Africa where fossils 4-8 million years old might be fou...
Campbell Biology: Concepts & Connections (9th Edition)
4. What five specific threats to biodiversity are described in this chapter? Provide an example of each.
Biology: Life on Earth (11th Edition)
In your own words, briefly distinguish between relative dates and numerical dates.
Applications and Investigations in Earth Science (9th Edition)
Fibrous connective tissue consists of ground substance and fibers that provide strength, support, and flexibili...
Human Biology: Concepts and Current Issues (8th Edition)
MAKE CONNECTIONS In Concept 20.2, you learned about genome-wide association studies. Explain how these studies...
Campbell Biology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 1. Please predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers.arrow_forwardElectrochemistry. Briefly describe the Donnan potential.arrow_forwardIndicate what the Luther equation is used for?arrow_forward
- Indicate one aspect that benefits and another that makes it difficult to use the hydroquinone electrode to measure pH.arrow_forwardAt an electrified interface according to the Gouy-Chapman model, what types of interactions do NOT occur between the ions and the solvent according to this theory?arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. Hint: In this case you must choose the best answer to demonstrate the stereochemistry of H2 addition. 1.03 2. (CH3)2S BIZ CH₂OH 2. DMS KMnO4, NaOH ΖΗ Pd or Pt (catalyst) HBr 20 1 HBr ROOR (peroxide) HO H-SO HC 12 11 10 BH, THE 2. H2O2, NaOH Brz cold HI 19 18 17 16 MCPBA 15 14 13 A Br H₂O BH3⚫THF Brz EtOH Pd or Ni (catalyst) D₂ (deuterium) 1. Os04 2. H2O2 CH3CO3H (peroxyacid) 1. MCPBA 2. H₂O* H B + H H H "H C H H Darrow_forward
- Explain how Beer’s Law can be used to determine the concentration in a selected food sample. Provide examples.arrow_forwardExplain the importance of having a sampling plan with respect to food analysis. Explain the importance of having a sampling plan with respect to food analysis. Provide examples.arrow_forwardPlease predict the products for each of the following reactions. Clearly show the regiochemistry (Markovnikov vs anti-Markovnikov) and stereochemistry (syn- vs anti- or both). If a mixture of enantiomers is formed, please draw all the enantiomers. cold KMnO4, NaOH 2. DMS 1. 03 CH3OH Br2 1. 03 2. (CH3)2S H₂ Pd or Pt (catalyst) HBr 18 19 20 1 HBr ROOR (peroxide) H₂O H₂SO4 HCI HI 17 16 6 15 MCPBA 1. BH3 THF 2. H₂O2, NaOH 1. OsO4 2. H₂O₂ 110 CH3CO₂H (peroxyacid) 1. MCPBA 2. H₂O* Br2 H₂O BH3 THF B12 EtOH Pd or Ni (catalyst) D₂ (deuterium) Bra A B C D H OH H OH OH H OH α α α OH H OH OH фон d H "Harrow_forward
- Briefly indicate the models that describe the structure of the interface: Helmholtz-Perrin, Gouy-Chapman, Stern and Grahame models.arrow_forwardElectrochemistry. Briefly describe the Gibbs model and the Gibbs absorption equation.arrow_forwardThermodynamic analysis of electrified interfaces.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY