
Foundations of Materials Science and Engineering
6th Edition
ISBN: 9781259696558
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4.8, Problem 1KCP
To determine
Describe and illustrate the solidification process of a pure metal in terms of the nucleation and growth of crystals.
Expert Solution & Answer
Explanation of Solution
Show the solidification process of a pure metal as in Figure (1).
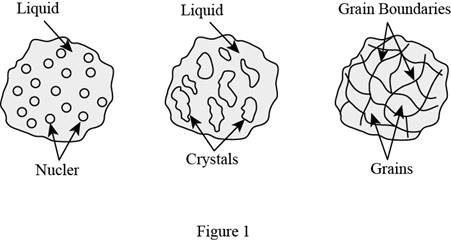
In general, the solidification process of a pure metal involves three stages in terms of the nucleation and growth of crystals. The first stage involves the development of stable nuclei in the fluid melt. The second stage involves the development of these cores or nuclei into stable crystals in the fluid melt and the last stage involves the development solidified structure enclosing grains formed from the crystals.
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
PROBLEM 3.23
3.23 Under normal operating condi-
tions a motor exerts a torque of
magnitude TF at F. The shafts
are made of a steel for which
the allowable shearing stress is
82 MPa and have diameters of
dCDE=24 mm and dFGH = 20
mm. Knowing that rp = 165
mm and rg114 mm, deter-
mine the largest torque TF
which may be exerted at F.
TF
F
rG-
rp
B
CH
TE
E
1. (16%) (a) If a ductile material fails under pure torsion, please explain the failure
mode and describe the observed plane of failure.
(b) Suppose a prismatic beam is subjected to equal and opposite couples as shown
in Fig. 1. Please sketch the deformation and the stress distribution of the cross
section.
M
M
Fig. 1
(c) Describe the definition of the neutral axis.
(d) Describe the definition of the modular ratio.
using the theorem of three moments, find all the moments, I only need concise calculations with minimal explanations. The correct answers are provided at the bottom
Chapter 4 Solutions
Foundations of Materials Science and Engineering
Ch. 4.8 - Prob. 1KCPCh. 4.8 - Define the homogeneous nucleation process for the...Ch. 4.8 - In the solidification of a pure metal, what are...Ch. 4.8 - In the solidification of a metal, what is the...Ch. 4.8 - During solidification, how does the degree of...Ch. 4.8 - Distinguish between homogeneous and heterogeneous...Ch. 4.8 - Describe the grain structure of a metal ingot that...Ch. 4.8 - Distinguish between equiaxed and columnar grains...Ch. 4.8 - How can the grain size of a cast ingot be refined?...Ch. 4.8 - Prob. 10KCP
Ch. 4.8 - Prob. 11KCPCh. 4.8 - Prob. 12KCPCh. 4.8 - Distinguish between a substitutional solid...Ch. 4.8 - What are the conditions that are favorable for...Ch. 4.8 - Prob. 15KCPCh. 4.8 - Prob. 16KCPCh. 4.8 - Prob. 17KCPCh. 4.8 - Prob. 18KCPCh. 4.8 - Describe the structure of a grain boundary. Why...Ch. 4.8 - Describe and illustrate the following planar...Ch. 4.8 - Prob. 21KCPCh. 4.8 - Describe the optical metallography technique. What...Ch. 4.8 - Prob. 23KCPCh. 4.8 - Prob. 24KCPCh. 4.8 - Prob. 25KCPCh. 4.8 - Prob. 26KCPCh. 4.8 - Prob. 27KCPCh. 4.8 - Prob. 28KCPCh. 4.8 - Prob. 29KCPCh. 4.8 - Prob. 30KCPCh. 4.8 - Prob. 31KCPCh. 4.8 - Calculate the size (radius) of the critically...Ch. 4.8 - Prob. 33AAPCh. 4.8 - Prob. 34AAPCh. 4.8 - Calculate the number of atoms in a critically...Ch. 4.8 - Prob. 36AAPCh. 4.8 - Prob. 37AAPCh. 4.8 - Prob. 38AAPCh. 4.8 - Prob. 39AAPCh. 4.8 - Prob. 40AAPCh. 4.8 - Prob. 41AAPCh. 4.8 - Prob. 42AAPCh. 4.8 - Determine, by counting, the ASTM grain-size number...Ch. 4.8 - Prob. 44AAPCh. 4.8 - For the grain structure in Problem 4.43, estimate...Ch. 4.8 - Prob. 46AAPCh. 4.8 - Prob. 47SEPCh. 4.8 - Prob. 48SEPCh. 4.8 - Prob. 49SEPCh. 4.8 - Prob. 50SEPCh. 4.8 - In Chapter 3 (Example Problem 3.11), we calculated...Ch. 4.8 - Prob. 52SEPCh. 4.8 - Prob. 53SEPCh. 4.8 - Prob. 54SEPCh. 4.8 - Prob. 55SEPCh. 4.8 - Prob. 56SEPCh. 4.8 - Prob. 57SEPCh. 4.8 - Prob. 58SEPCh. 4.8 - Prob. 59SEPCh. 4.8 - Prob. 60SEPCh. 4.8 - Prob. 61SEPCh. 4.8 - Prob. 62SEPCh. 4.8 - Prob. 63SEPCh. 4.8 - Prob. 64SEPCh. 4.8 - Prob. 65SEPCh. 4.8 - Prob. 66SEP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- PROBLEM 3.46 The solid cylindrical rod BC of length L = 600 mm is attached to the rigid lever AB of length a = 380 mm and to the support at C. When a 500 N force P is applied at A, design specifications require that the displacement of A not exceed 25 mm when a 500 N force P is applied at A For the material indicated determine the required diameter of the rod. Aluminium: Tall = 65 MPa, G = 27 GPa. Aarrow_forwardFind the equivalent mass of the rocker arm assembly with respect to the x coordinate. k₁ mi m2 k₁arrow_forward2. Figure below shows a U-tube manometer open at both ends and containing a column of liquid mercury of length l and specific weight y. Considering a small displacement x of the manometer meniscus from its equilibrium position (or datum), determine the equivalent spring constant associated with the restoring force. Datum Area, Aarrow_forward
- 1. The consequences of a head-on collision of two automobiles can be studied by considering the impact of the automobile on a barrier, as shown in figure below. Construct a mathematical model (i.e., draw the diagram) by considering the masses of the automobile body, engine, transmission, and suspension and the elasticity of the bumpers, radiator, sheet metal body, driveline, and engine mounts.arrow_forward3.) 15.40 – Collar B moves up at constant velocity vB = 1.5 m/s. Rod AB has length = 1.2 m. The incline is at angle = 25°. Compute an expression for the angular velocity of rod AB, ė and the velocity of end A of the rod (✓✓) as a function of v₂,1,0,0. Then compute numerical answers for ȧ & y_ with 0 = 50°.arrow_forward2.) 15.12 The assembly shown consists of the straight rod ABC which passes through and is welded to the grectangular plate DEFH. The assembly rotates about the axis AC with a constant angular velocity of 9 rad/s. Knowing that the motion when viewed from C is counterclockwise, determine the velocity and acceleration of corner F.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Welding: Principles and Applications (MindTap Cou...Mechanical EngineeringISBN:9781305494695Author:Larry JeffusPublisher:Cengage Learning
Welding: Principles and Applications (MindTap Cou...Mechanical EngineeringISBN:9781305494695Author:Larry JeffusPublisher:Cengage Learning

Welding: Principles and Applications (MindTap Cou...
Mechanical Engineering
ISBN:9781305494695
Author:Larry Jeffus
Publisher:Cengage Learning