
Concept explainers
Interpretation: The product formed after the crossed-aldol condensation between 2-nitrobenzaldehyde and acetone in the first step for synthesis of indigo needs to be determined.
Concept Introduction:In crossed-aldol condensation, two different carbonyl compounds (both with alpha hydrogen atom) undergo condensation reaction together. There is a possibility of 4 products in such type of condensation reaction.
Explanation of Solution
The formation of indigo is represented as follows:
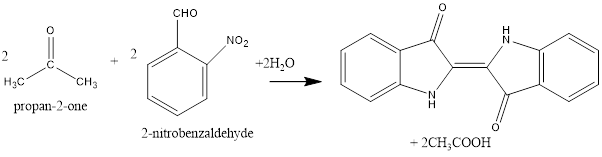
The first step of the reaction is crossed aldol condensation. The given reactants are 2-nitrobenzaldehyde and acetone or propan-2-one. Both of the reactantshasa carbonyl group thus, both are carbonyl compounds. Since, both the carbonyl compounds are different thus, crossed aldol condensation is possible.
The structure of reactants is represented as follows:
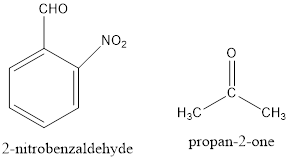
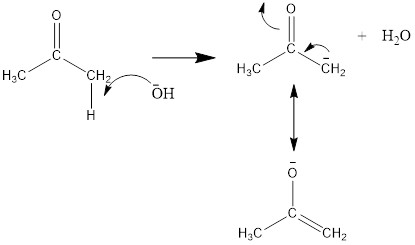
Here, the removal of alpha hydrogen from 2-nitrobenzaldehyde is not possible because the negative charge on the double bond is not stable. Thus, the hydroxide ion of water removes the alpha hydrogen of acetone or propan-2-one.
The mechanism for the crossed aldol condensation will be as follows:
Now, the negative charge will attack the carbonyl group of 2-nitrobenzaldehyde as follows and results in the formation of a hydroxy
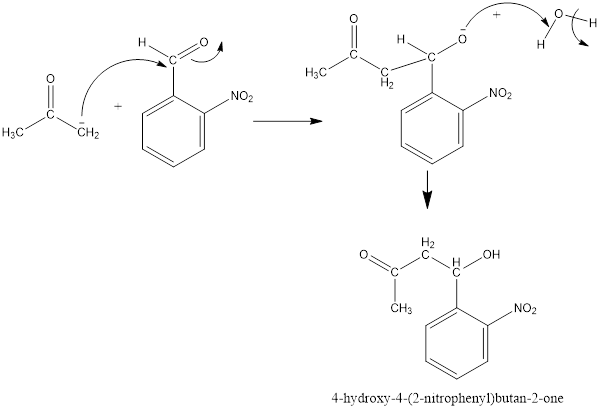
Thus, the product formed in the first step will be as follows:
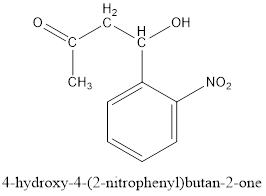
After this series of condensations, tautomerizations, a retro Claisen condensation and elimination reactions result in the formation of indigo.
Want to see more full solutions like this?
Chapter 45 Solutions
A Small Scale Approach to Organic Laboratory Techniques
- Synthesize 2-Ethyl-3-methyloxirane from dimethyl(propyl)sulfonium iodide using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize 2-Hydroxy-2-phenylacetonitrile from phenylmethanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardSynthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Synthesize N-Methylcyclohexylamine from cyclohexanol using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forwardIf possible, please provide the formula of the compound 3,3-dimethylbut-2-enal.arrow_forwardSynthesize 1,4-dibromobenzene from acetanilide (N-phenylacetamide) using the necessary organic or inorganic reagents. Draw the structures of the compounds.arrow_forward
- Indicate the products obtained by mixing (3-oxo-3-phenylpropyl)triphenylphosphonium bromide with sodium hydride.arrow_forwardWe mix N-ethyl-2-hexanamine with excess methyl iodide and followed by heating with aqueous Ag2O. Indicate the major products obtained.arrow_forwardIndicate the products obtained by mixing acetophenone with iodine and NaOH.arrow_forward
- Indicate the products obtained by mixing 2-Propanone and ethyllithium and performing a subsequent acid hydrolysis.arrow_forwardIndicate the products obtained if (E)-2-butenal and 3-oxo-butanenitrile are mixed with sodium ethoxide in ethanol.arrow_forwardQuestion 3 (4 points), Draw a full arrow-pushing mechanism for the following reaction Please draw all structures clearly. Note that this intramolecular cyclization is analogous to the mechanism for halohydrin formation. COH Br + HBr Brarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


