
Chemistry
12th Edition
ISBN: 9780078021510
Author: Raymond Chang Dr., Kenneth Goldsby Professor
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4.3, Problem 1RC
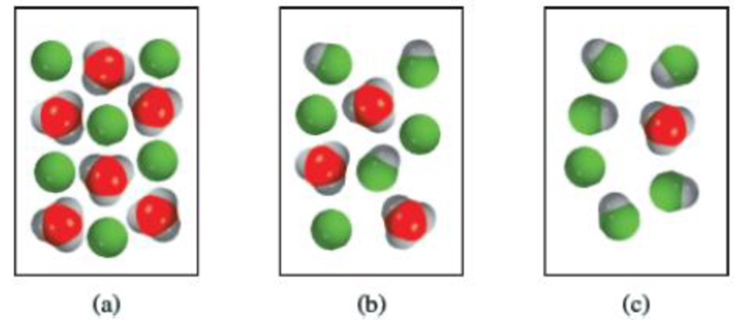
Which of the diagrams (a)–(c) best represents a weak acid? Which represents a very weak acid? Which represents a strong acid? The proton exists in water as the hydronium ion. All acids are monoprotic. (For simplicity, water molecules are not shown.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A block of zinc has an initial temperature of 94.2 degrees celcius and is immererd in 105 g of water at 21.90 degrees celcius. At thermal equilibrium, the final temperature is 25.20 degrees celcius. What is the mass of the zinc block? Cs(Zn) = 0.390 J/gxdegrees celcius Cs(H2O) = 4.18 J/gx degrees celcus
Potential Energy (kJ)
1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction.
AH = -950 kJ
AH = 575 kJ
(i) Cl₂ (g) + Pt (s) 2C1 (g) + Pt (s)
Ea = 1550 kJ
(ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s)
(iii) Cl (g) + CICO (g) → Cl₂CO (g)
Ea = 2240 kJ
Ea = 2350 kJ
AH = -825 kJ
2600
2400
2200
2000
1800
1600
1400
1200
1000
a. Draw the potential energy diagram for the reaction. Label the data points for clarity.
The potential energy of the reactants is 600 kJ
800
600
400
200
0
-200-
-400
-600-
-800-
Reaction Progress
Can u help me figure out the reaction mechanisms for these, idk where to even start
Chapter 4 Solutions
Chemistry
Ch. 4.1 - Prob. 1RCCh. 4.2 - Classify the following ionic compounds as soluble...Ch. 4.2 - Predict the precipitate produced by mixing an...Ch. 4.2 - Which of the diagrams (a)(c) accurately describes...Ch. 4.3 - Which of the diagrams (a)(c) best represents a...Ch. 4.3 - Classify each of the following species as a...Ch. 4.3 - Write a molecular equation, an ionic equation, and...Ch. 4.4 - Assign oxidation numbers to all the elements in...Ch. 4.4 - Prob. 6PECh. 4.4 - Which of the following combination reactions is...
Ch. 4.5 - Prob. 7PECh. 4.5 - Prob. 8PECh. 4.5 - Prob. 9PECh. 4.5 - Prob. 1RCCh. 4.6 - A sample of 0.3220 g of an ionic compound...Ch. 4.6 - Prob. 1RCCh. 4.7 - How many grams of KHP are needed to neutralize...Ch. 4.7 - Prob. 12PECh. 4.8 - Prob. 13PECh. 4.8 - If a solution of a reducing agent is titrated with...Ch. 4 - Define solute, solvent, and solution by describing...Ch. 4 - What is the difference between a nonelectrolyte...Ch. 4 - Describe hydration. What properties of water...Ch. 4 - What is the difference between the following...Ch. 4 - Water is an extremely weak electrolyte and...Ch. 4 - Sodium sulfate (Na2SO4) is a strong electrolyte....Ch. 4 - Prob. 4.7QPCh. 4 - Prob. 4.8QPCh. 4 - Identify each of the following substances as a...Ch. 4 - Identify each of the following substances as a...Ch. 4 - The passage of electricity through an electrolyte...Ch. 4 - Predict and explain which of the following systems...Ch. 4 - You are given a water-soluble compound X. Describe...Ch. 4 - Explain why a solution of HCl in benzene does not...Ch. 4 - What is the difference between an ionic equation...Ch. 4 - What is the advantage of writing net ionic...Ch. 4 - Two aqueous solutions of AgNO3 and NaCl are mixed....Ch. 4 - Two aqueous solutions of KOH and MgCl2 are mixed....Ch. 4 - Characterize the following compounds as soluble or...Ch. 4 - Characterize the following compounds as soluble or...Ch. 4 - Write ionic and net ionic equations for the...Ch. 4 - Write ionic and net ionic equations for the...Ch. 4 - Which of the following processes will likely...Ch. 4 - Prob. 4.24QPCh. 4 - List the general properties of acids and bases.Ch. 4 - Give Arrheniuss and Brnsteds definitions of an...Ch. 4 - Give an example of a monoprotic acid, a diprotic...Ch. 4 - What are the characteristics of an acid-base...Ch. 4 - What factors qualify a compound as a salt? Specify...Ch. 4 - Prob. 4.30QPCh. 4 - Prob. 4.31QPCh. 4 - Identify each of the following species as a...Ch. 4 - Balance the following equations and write the...Ch. 4 - Balance the following equations and write the...Ch. 4 - Prob. 4.35QPCh. 4 - True or false: All combustion reactions are redox...Ch. 4 - Prob. 4.37QPCh. 4 - Prob. 4.38QPCh. 4 - How is the activity series organized? How is it...Ch. 4 - Use the following reaction to define redox...Ch. 4 - Prob. 4.41QPCh. 4 - What is the requirement for an element to undergo...Ch. 4 - For the complete redox reactions given here, (i)...Ch. 4 - Prob. 4.44QPCh. 4 - Arrange the following species in order of...Ch. 4 - Phosphorus forms many oxoacids. Indicate the...Ch. 4 - Give the oxidation number of the underlined atoms...Ch. 4 - Give the oxidation number for the following...Ch. 4 - Give oxidation number for the underlined atoms in...Ch. 4 - Give the oxidation number of the underlined atoms...Ch. 4 - Nitric acid is a strong oxidizing agent. State...Ch. 4 - Which of the following metals can react with...Ch. 4 - On the basis of oxidation number considerations,...Ch. 4 - Predict the outcome of the reactions represented...Ch. 4 - Classify the following redox reactions. (a)...Ch. 4 - Classify the following redox reactions. (a)...Ch. 4 - Which of the following are redox processes?...Ch. 4 - Of the following, which is most likely to be the...Ch. 4 - Write the equation for calculating molarity. Why...Ch. 4 - Describe the steps involved in preparing a...Ch. 4 - Calculate the mass of KI in grams required to...Ch. 4 - Describe how you would prepare 250 mL of a 0.707 M...Ch. 4 - Prob. 4.63QPCh. 4 - Prob. 4.64QPCh. 4 - Calculate the molarity of each of the following...Ch. 4 - Calculate the molarity of each of the following...Ch. 4 - Calculate the volume in milliliters of a solution...Ch. 4 - Prob. 4.68QPCh. 4 - What volume of 0.416 M Mg(NO3)2 should be added to...Ch. 4 - Barium hydroxide, often used to titrate weak...Ch. 4 - Describe the basic steps involved in diluting a...Ch. 4 - Write the equation that enables us to calculate...Ch. 4 - Describe how to prepare 1.00 L of 0.646 M HCl...Ch. 4 - Water is added to 25.0 mL of a 0.866 M KNO3...Ch. 4 - How would you prepare 60.0 mL of 0.200 M HNO3 from...Ch. 4 - You have 505 mL of a 0.125 M HCl solution and you...Ch. 4 - A 35.2-mL, 1.66 M KMnO4 solution is mixed with...Ch. 4 - A 46.2-mL, 0.568 M calcium nitrate [Ca(NO3)2]...Ch. 4 - Describe the basic steps involved in gravimetric...Ch. 4 - Distilled water must be used in the gravimetric...Ch. 4 - If 30.0 mL of 0.150 M CaCl2 is added to 15.0 mL of...Ch. 4 - A sample of 0.6760 g of an unknown compound...Ch. 4 - How many grams of NaCl are required to precipitate...Ch. 4 - The concentration of sulfate in water can be...Ch. 4 - Describe the basic steps involved in an acid-base...Ch. 4 - How does an acid-base indicator work?Ch. 4 - Prob. 4.87QPCh. 4 - Would the volume of a 0.10 M NaOH solution needed...Ch. 4 - A quantity of 18.68 mL of a KOH solution is needed...Ch. 4 - Calculate the concentration (in molarity) of a...Ch. 4 - Calculate the volume in milliliters of a 1.420 M...Ch. 4 - What volume of a 0.500 M HCl solution is needed to...Ch. 4 - What are the similarities and differences between...Ch. 4 - Explain why potassium permanganate (KMnO4) and...Ch. 4 - Iron(II) can be oxidized by an acidic K2Cr2O7...Ch. 4 - The SO2 present in air is mainly responsible for...Ch. 4 - Prob. 4.97QPCh. 4 - The concentration of a hydrogen peroxide solution...Ch. 4 - Oxalic acid (H2C2O4) is present in many plants and...Ch. 4 - Prob. 4.100QPCh. 4 - Iodate ion, IO3, oxidizes SO32 in acidic solution....Ch. 4 - Calcium oxalate (CaC2O4), the main component of...Ch. 4 - Prob. 4.103QPCh. 4 - Prob. 4.104QPCh. 4 - Prob. 4.105QPCh. 4 - A 5.00 102 mL sample of 2.00 M HCl solution is...Ch. 4 - Shown are two aqueous solutions containing various...Ch. 4 - Shown are two aqueous solutions containing various...Ch. 4 - Calculate the volume of a 0.156 M CuSO4 solution...Ch. 4 - Prob. 4.110QPCh. 4 - A 3.664-g sample of a monoprotic acid was...Ch. 4 - Prob. 4.112QPCh. 4 - A 15.00-mL solution of potassium nitrate (KNO3)...Ch. 4 - When a 2.50-g zinc strip was placed in a AgNO3...Ch. 4 - Calculate the mass of the precipitate formed when...Ch. 4 - Calculate the concentration of the acid (or base)...Ch. 4 - (a) Describe a preparation for magnesium hydroxide...Ch. 4 - A 1.00-g sample of a metal X (that is known to...Ch. 4 - Prob. 4.119QPCh. 4 - The molecular formula of malonic acid is C3H4O4....Ch. 4 - Prob. 4.121QPCh. 4 - A 60.0-mL 0.513 M glucose (C6H12O6) solution is...Ch. 4 - An ionic compound X is only slightly soluble in...Ch. 4 - Prob. 4.124QPCh. 4 - Prob. 4.125QPCh. 4 - Prob. 4.126QPCh. 4 - The molar mass of a certain metal carbonate, MCO3,...Ch. 4 - Prob. 4.128QPCh. 4 - You are given a soluble compound of unknown...Ch. 4 - Prob. 4.130QPCh. 4 - Prob. 4.131QPCh. 4 - Prob. 4.132QPCh. 4 - Prob. 4.133QPCh. 4 - Prob. 4.134QPCh. 4 - Prob. 4.135QPCh. 4 - Prob. 4.136QPCh. 4 - Describe in each case how you would separate the...Ch. 4 - Prob. 4.138QPCh. 4 - Prob. 4.139QPCh. 4 - A 0.8870-g sample of a mixture of NaCl and KCl is...Ch. 4 - Prob. 4.141QPCh. 4 - Prob. 4.142QPCh. 4 - Prob. 4.143QPCh. 4 - A useful application of oxalic acid is the removal...Ch. 4 - Prob. 4.145QPCh. 4 - A 0.9157-g mixture of CaBr2 and NaBr is dissolved...Ch. 4 - Prob. 4.147QPCh. 4 - A 325-mL sample of solution contains 25.3 g of...Ch. 4 - Prob. 4.149QPCh. 4 - Prob. 4.150QPCh. 4 - Prob. 4.151QPCh. 4 - Prob. 4.152QPCh. 4 - Prob. 4.153QPCh. 4 - Prob. 4.154QPCh. 4 - Prob. 4.155QPCh. 4 - Prob. 4.156QPCh. 4 - Prob. 4.157QPCh. 4 - Prob. 4.158QPCh. 4 - Prob. 4.159QPCh. 4 - Prob. 4.160QPCh. 4 - The following cycle of copper experiment is...Ch. 4 - A quantity of 25.0 mL of a solution containing...Ch. 4 - Prob. 4.163QPCh. 4 - Prob. 4.165QPCh. 4 - Prob. 4.166QPCh. 4 - Prob. 4.167QPCh. 4 - Many proteins contain metal ions for structural...Ch. 4 - Prob. 4.170IMECh. 4 - Prob. 4.171IMECh. 4 - Prob. 4.172IMECh. 4 - Muriatic acid, a commercial-grade hydrochloric...Ch. 4 - Because acid-base and precipitation reactions...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi, I need your help with the drawing, please. I have attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forwardHi, I need your help i dont know which one to draw please. I’ve attached the question along with my lab instructions. Please use the reaction from the lab only, as we are not allowed to use outside sources. Thank you!arrow_forward5. Write the formation reaction of the following complex compounds from the following reactants: 6. AgNO₃ + K₂CrO₂ + NH₄OH → 7. HgNO₃ + excess KI → 8. Al(NO₃)₃ + excess NaOH →arrow_forward
- Indicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. CO₂C2H5 + CH3-NH-NH,arrow_forwardDraw the major product of this reaction N-(cyclohex-1-en-1-yl)-1-(pyrrolidino) reacts with CH2=CHCHO, heat, H3O+arrow_forwardDraw the starting material that would be needed to make this product through an intramolecular Dieckmann reactionarrow_forward
- Draw the major product of this reaction. Nitropropane reacts + pent-3-en-2-one reacts with NaOCH2CH3, CH3CHOHarrow_forwardIndicate whether the product formed in the reaction exhibits tautomerism. If so, draw the structure of the tautomers. OC2H5 + CoHs-NH-NH,arrow_forwardExplain how substitutions at the 5-position of barbituric acid increase the compound's lipophilicity.arrow_forward
- Explain how substitutions at the 5-position of phenobarbital increase the compound's lipophilicity.arrow_forwardName an interesting derivative of barbituric acid, describing its structure.arrow_forwardBriefly describe the synthesis mechanism of barbituric acid from the condensation of urea with a β-diketone.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY