![OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months)](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months)
11th Edition
ISBN: 9781305106734
Author: Frederick A. Bettelheim; William H. Brown; Mary K. Campbell; Shawn O. Farrell; Omar Torres
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4.2, Problem 4.1P
Problem 4-1 Following is an unbalanced equation for photosynthesis, the process by which green plants convert carbon dioxide and water to glucose and oxygen. Balance this equation:
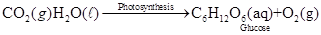
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Calculate the proton and carbon chemical shifts for this structure
A.
B.
b. Now consider the two bicyclic molecules A. and B. Note that A. is a dianion
and B. is a neutral molecule. One of these molecules is a highly reactive
compound first characterized in frozen noble gas matrices, that self-reacts
rapidly at temperatures above liquid nitrogen temperature. The other
compound was isolated at room temperature in the early 1960s, and is a
stable ligand used in organometallic chemistry. Which molecule is the more
stable molecule, and why?
Where are the chiral centers in this molecule? Also is this compound meso yes or no?
Chapter 4 Solutions
OWLv2 for Bettelheim/Brown/Campbell/Farrell/Torres' Introduction to General, Organic and Biochemistry, 11th Edition, [Instant Access], 1 term (6 months)
Ch. 4.2 - Problem 4-1 Following is an unbalanced equation...Ch. 4.2 - Problem 4-2 Balance this equation:Ch. 4.2 - Prob. 4.3PCh. 4.3 - Problem 4-4 When a solution of copper(II)...Ch. 4.4 - Problem 4-5 In each equation, identify the...Ch. 4.5 - Problem 4-6 What is (a) the molecular weight of...Ch. 4.6 - Prob. 4.7PCh. 4.6 - Problem 4-8 We wish to weigh 2.84 mol of sodium...Ch. 4.6 - Problem 4-9 How many moles of C atoms, H atoms,...Ch. 4.6 - Problem 4-10 How many moles of copper(I) ions,...
Ch. 4.6 - Prob. 4.11PCh. 4.7 - Prob. 4.12PCh. 4.7 - Prob. 4.13PCh. 4.7 - Problem 4-14 Ethanol is produced industrially by...Ch. 4.7 - Prob. 4.15PCh. 4.7 - Prob. 4.16PCh. 4 - 4-17 Balance each equation.Ch. 4 - 4-18 Balance each equation.Ch. 4 - Prob. 4.19PCh. 4 - 4-20 Calcium oxide is prepared by heating...Ch. 4 - 4-21 The brilliant white light in some firework...Ch. 4 - Prob. 4.22PCh. 4 - 4-23 When solid carbon burns in a limited supply...Ch. 4 - Prob. 4.24PCh. 4 - 4-25 In the chemical test for arsenic, the gas...Ch. 4 - Prob. 4.26PCh. 4 - Prob. 4.27PCh. 4 - 4-28 Answer true or false. (a) A net ionic...Ch. 4 - 4-29 Balance these net ionic equations. (a)...Ch. 4 - 4-30 In the equation (a) Identify the spectator...Ch. 4 - 4-31 Predict whether a precipitate will form when...Ch. 4 - 4-32 When a solution of ammonium chloride is added...Ch. 4 - 4-33 When a solution of hydrochloric acid, HCl, is...Ch. 4 - Prob. 4.34PCh. 4 - Prob. 4.35PCh. 4 - 4-36 Using the solubility generalizations given in...Ch. 4 - 4-37 Answer true or false. (a) When a substance is...Ch. 4 - Prob. 4.38PCh. 4 - Prob. 4.39PCh. 4 - Prob. 4.40PCh. 4 - Prob. 4.41PCh. 4 - 4-42 Calculate the formula weight of: (a) KCl (b)...Ch. 4 - 4-43 Calculate the molecular weight of: (a)...Ch. 4 - 4-44 Answer true or false. (a) The mole is a...Ch. 4 - 4-45 Calculate the number of moles in: (a) 32 g of...Ch. 4 - 4-46 Calculate the number of grams in: (a) 1.77...Ch. 4 - 4-47 Calculate the number of moles of: (a) O atoms...Ch. 4 - 4-48 Calculate the number of moles of: (a) S2-...Ch. 4 - 4-49 Calculate the number of: (a) nitrogen atoms...Ch. 4 - 4-50 How many molecules are in each of the...Ch. 4 - 4-51 What is the mass in grams of each number of...Ch. 4 - 4-52 The molecular weight of hemoglobin is about...Ch. 4 - 4-53 A typical deposit of cholesterol, C27H46O, in...Ch. 4 - 4-54 Answer true or false. (a) Stoichiometry is...Ch. 4 - 4-55 For the reaction: (a) How many moles of N2...Ch. 4 - 4-56 Magnesium reacts with sulfuric acid according...Ch. 4 - 4-57 Chloroform, CHCl3, is prepared industrially...Ch. 4 - 4-58 At one time, acetaldehyde was prepared...Ch. 4 - 4-59 Chlorine dioxide, ClO2, is used for bleaching...Ch. 4 - 4-60 Ethanol, C2H6O, is added to gasoline to...Ch. 4 - 4-61 In photosynthesis, green plants convert CO2...Ch. 4 - 4-62 Iron ore is converted to iron by heating it...Ch. 4 - Prob. 4.63PCh. 4 - 4-64 Aspirin is made by the reaction of salicylic...Ch. 4 - 4-65 Suppose the preparation of aspirin from...Ch. 4 - 4-66 Benzene reacts with bromine to produce...Ch. 4 - 4-67 Ethyl chloride is prepared by the reaction of...Ch. 4 - 4-68 Diethyl ether is made from ethanol according...Ch. 4 - Prob. 4.69PCh. 4 - Prob. 4.70PCh. 4 - 4-71 Which of these reactions are exothermic, and...Ch. 4 - Prob. 4.72PCh. 4 - Prob. 4.73PCh. 4 - Prob. 4.74PCh. 4 - Prob. 4.75PCh. 4 - Prob. 4.76PCh. 4 - 4-77 To convert 1 mol of iron(III) oxide to its...Ch. 4 - 4-78 (Chemical Connections 4A) How does fluoride...Ch. 4 - Prob. 4.79PCh. 4 - Prob. 4.80PCh. 4 - 4-81 (Chemical Connections 4C) Balance the lithium...Ch. 4 - 4-82 When gaseous dinitrogen pentoxide, N2O5, is...Ch. 4 - Prob. 4.83PCh. 4 - Prob. 4.84PCh. 4 - Prob. 4.85PCh. 4 - 4-86 When an aqueous solution of Na3PO4 is added...Ch. 4 - Prob. 4.87PCh. 4 - 4-88 Chlorophyll, the compound responsible for the...Ch. 4 - 4-89 If 7.0 kg of is added to 11.0 kg of to form...Ch. 4 - 4-90 Lead(lI) nitrate and aluminum chloride react...Ch. 4 - 4-91 Assume that the average red blood cell has a...Ch. 4 - 4-92 Reaction of pentane, C5H12, with oxygen, O2,...Ch. 4 - 4-93 Ammonia is prepared industrially by the...Ch. 4 - 4-94 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is...Ch. 4 - Prob. 4.95PCh. 4 - Prob. 4.96PCh. 4 - Prob. 4.97PCh. 4 - Prob. 4.98PCh. 4 - Prob. 4.99PCh. 4 - Prob. 4.100PCh. 4 - Prob. 4.101PCh. 4 - 4-102 Aspartame, an artificial sweetener used as a...Ch. 4 - 4-103 Caffeine, a central nervous system...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- PLEASE HELP! URGENT!arrow_forwardWhere are the chiral centers in this molecule? Also is this compound meso yes or no?arrow_forwardA mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forward
- How many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forwardHow many chiral carbons are in the molecule? Farrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32; Author: Crash Course;https://www.youtube.com/watch?v=7qOFtL3VEBc;License: Standard YouTube License, CC-BY