
(a)
Interpretation:
The systematic name for the following compound should be determined.
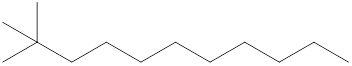
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(b)
Interpretation:
The systematic name for the following compound should be determined.
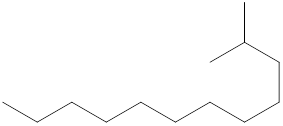
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(c)
Interpretation:
The systematic name for the following compound should be determined.
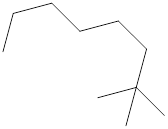
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(d)
Interpretation:
The systematic name for the following compound should be determined.
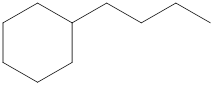
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
The compounds in which a series of atoms are connected to form a ring is known as cyclic compound whereas the compounds which are open chain compounds and their atoms don't form a ring is known as acyclic compounds. The general molecular formula of a cyclic alkane is
Rules of naming cycloalkanes are:
- First, determine the cycloalkane present in the structure which is considered as a parent chain (maximum number of carbon atoms). If the acyclic alkane chain has more carbon atoms, then the alkyl chain is considered a parent chain.
- For a cyclic system, the number of carbon atoms must be identified as present in different paths connected with two bridgeheads.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group or cycloalkyl group and denote its position on the parent chain with the number
- The alkyl groups or cycloalkyl groups are written in alphabetical order.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ORGANIC CHEMISTRY
- A mixture of C7H12O2, C9H9OCl, biphenyl and acetone was put together in a gas chromatography tube. Please decide from the GC resutls which correspond to the peak for C7,C9 and biphenyl and explain the reasoning based on GC results. Eliminate unnecessary peaks from Gas Chromatography results.arrow_forwardIs the molecule chiral, meso, or achiral? CI .CH3 H₂C CIarrow_forwardPLEASE HELP ! URGENT!arrow_forward
- Identify priority of the substituents: CH3arrow_forwardHow many chiral carbons are in the molecule? OH F CI Brarrow_forwardA mixture of three compounds Phen-A, Acet-B and Rin-C was analyzed using TLC with 1:9 ethanol: hexane as the mobile phase. The TLC plate showed three spots of R, 0.1 and 0.2 and 0.3. Which of the three compounds (Phen-A; Acet-B or Rin-C) would have the highest (Blank 1), middle (Blank 2) and lowest (Blank 3) spot respectively? 0 CH: 0 CH, 0 H.C OH H.CN OH Acet-B Rin-C phen-A A A <arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT





