
4-103 Caffeine, a central nervous system stimulant, has the molecular formula C8H10N402.
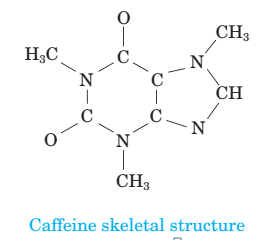
(a) How many moles of caffeine are present in 6.19 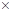 1025 molecules of caffeine?
1025 molecules of caffeine?
(b) Imagine you dissolve caffeine in water to a volume of 100.0 mL, which is known to have a density of 1.23 g/mL. How many molecules of caffeine are present in this volume?
(c) How many nitrogen atoms are present in 3.5 mg of caffeine?
(d) Complete the skeletal structure of caffeine, where all the bonded atoms are shown but double bonds, triple bonds, and/or lone pairs are missing.
(e) Identify the various types of geometries present in each central atom of caffeine using VSEPR theory.
(f) Determine the various relative bond angles associated with each central atom of caffeine using VSEPR theory
(g) What is the most polar bond in caffeine?
(h) Would you predict caffeine to be polar or nonpolar?
(j) Consider the combustion of caffeine, which results in formation of NO2(g) as well as other expected products. Write a balanced chemical equation for this reaction.
(j) The heat of combustion for caffeine is 2211 kcall/mol. How much heat will be given off if 0.81 g of caffeine is burned completely?
(k) Calculate the weight of H2O(g) that can be prepared from 8.00 g of caffeine mixed with 20.3 g of oxygen gas.
(a)
Interpretation:
Calculate the number of moles of caffeine in 6.19×1025 molecules.
Concept Introduction:
The caffeine is a chemical, which is found abundantly in some beverage like tea, coffee, cola and mate etc. It is known to be a nervous system stimulant and used to improve the alertness of the brain.
According to Avogadro’s law, the number of units in 1 mol of a substance is equal to 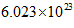 This is known as Avogadro’s number.
This is known as Avogadro’s number.
Answer to Problem 93P
6.19×1025 molecules are equal to 103 moles of caffeine.
Explanation of Solution
The number of moles present in 6.19×1025 molecules of caffeine can be calculated as follows:
(b)
Interpretation:
Calculate the number of molecules of caffeine in a solution, which has a density 1.23g/ml.
Concept Introduction:
The caffeine is a chemical, which is found abundantly in some beverage like tea, coffee, cola and mate etc. It is known to be a nervous system stimulant and used to improve the alertness of the brain.
Density of a substance is related to its mass and volume as follows:
From mass and molar mass, number of moles of substance can be calculated as follows:
Here, n is number of moles, m is mass and m is molar mass.
Also, according to Avogadro’s law, the number of units in 1 mol of a substance is equal to
Answer to Problem 93P
The number of molecules of caffeine in the solution is 3.82×1023.
Explanation of Solution
The number of molecules present in 100ml solution of a density1.23g/ml can be calculated as follows:
Mass of 1 mole of caffeine is.
(c)
Interpretation:
Calculate the number of molecules of nitrogen atoms in 3.5 mg of caffeine.
Concept Introduction:
The caffeine is a chemical, which is found abundantly in some beverage like tea, coffee, cola and mate etc. It is known to be a nervous system stimulant and used to improve the alertness of the brain.
From mass and molar mass, number of moles of substance can be calculated as follows:
Here, n is number of moles, m is mass and m is molar mass.
Also, according to Avogadro’s law, the number of units in 1 mol of a substance is equal to 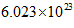 This is known as Avogadro’s number.
This is known as Avogadro’s number.
Answer to Problem 93P
The number of nitrogen atom in 3.5mg caffeine is4.32×1022.
Explanation of Solution
The number of nitrogen atom in 3.5mg caffeine can be calculated as follows:
(d)
Interpretation:
Describe the skeletal structure of the caffeine.
Concept Introduction:
The caffeine is a chemical, which is found abundantly in some beverage like tea, coffee, cola and mate etc. It is known to be a nervous system stimulant and used to improve the alertness of the brain.
Answer to Problem 93P
The skeletal structure of the caffeine is.
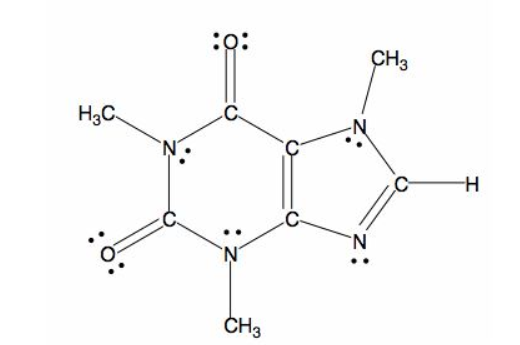
Explanation of Solution
All caffeine is made up of two carbon rings, one has 4 carbon and other has 3. The ring is joined by a two shared carbon atom united by the double bond. The Lewis dot structure of the caffeine is depicted below.
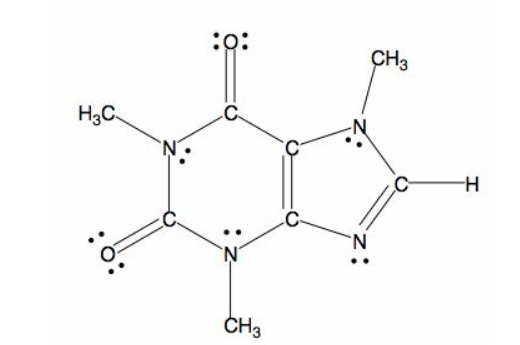
(e)
Interpretation:
Describe the geometric details of the central atom of the caffeine.
Concept Introduction:
The caffeine is a chemical, which is found abundantly in some beverage like tea, coffee, cola and mate etc. It is known to be a nervous system stimulant and used to improve the alertness of the brain.
Answer to Problem 93P
The detailed geometric analysis of the central atom if the caffeine is described below.
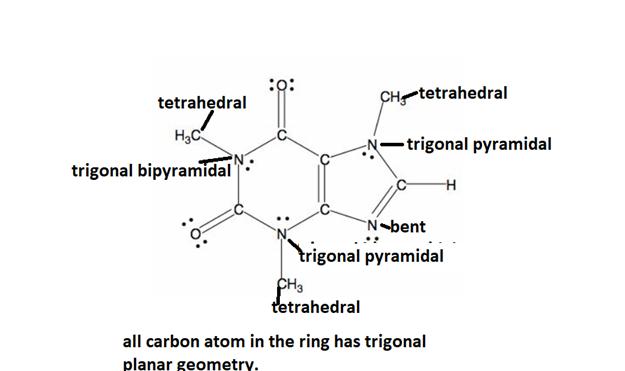
Explanation of Solution
All caffeine is made up of two carbon rings, one has 4 carbon and other has 3. The ring is joined by a two shared carbon atom united by the double bond. Geometry exhibited by the caffeine’s central molecules is described below:
(f)
Interpretation:
Describe the bond angles of the caffeine.
Concept Introduction:
The caffeine is a chemical, which is found abundantly in some beverage like tea, coffee, cola and mate etc. It is known to be a nervous system stimulant and used to improve the alertness of the brain.
Answer to Problem 93P
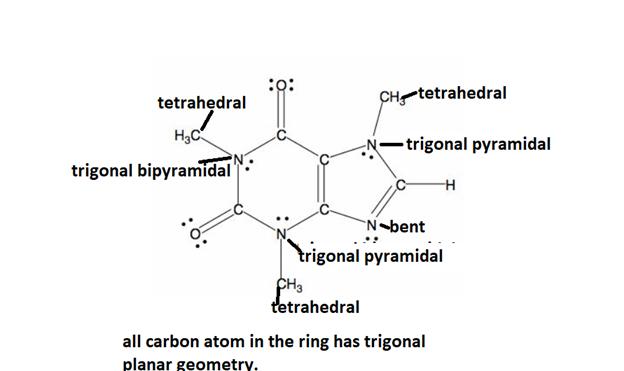
The detailed bond angle analysis of the caffeine is given below.
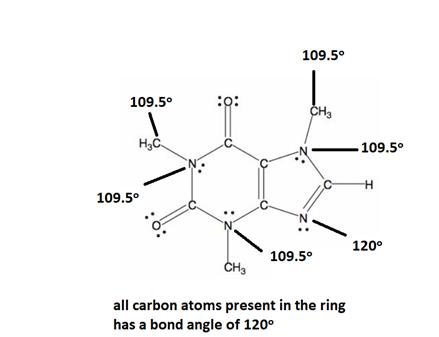
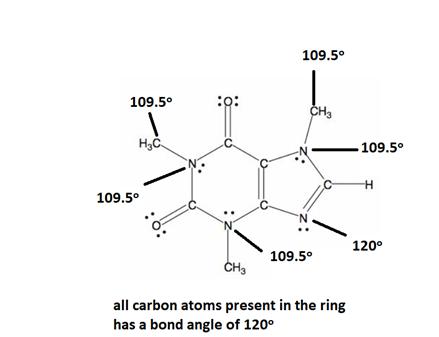
Explanation of Solution
All caffeine is made up of two carbon rings, one has 4 carbon and other has 3. All tetrahedral and the trigonal planar structure have an angle of 109.5° and the bent nitrogen atom exhibit a bond angle of 120°. all the carbon atom in the ring has a bond angle of 1200.
(g)
Interpretation:
Describe the most polar bond in the caffeine molecule.
Concept Introduction:
The polarity in bond is due to difference in electronegitivity of atoms joined together via bond. The atom which is more electronegativity gets a partial negative charge and atom which is less electronegative or electropositive in nature gets a partial positive charge.
Answer to Problem 93P
C-O bonds.
Explanation of Solution
The structure of caffeine is as follows:
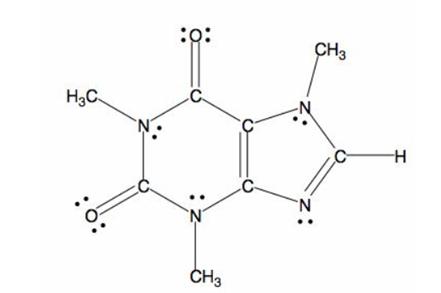
The bond formed between electronegative and electropositive atom will be polar in nature. Here, nitrogen and oxygen atoms are two electronegative atoms and carbon is electropositive atom. Thus, the C-N and C-O bonds will be polar in nature.
Since, oxygen is more electronegative than nitrogen, it gets more partial negative charge and the C-O bonds will be more polar.
(h)
Interpretation:
If the caffeine molecule is polar or non-polar should be determined.
Concept Introduction:
The polarity in bond is due to difference in electronegitivity of atoms joined together via bond. The atom which is more electronegativity gets a partial negative charge and atom which is less electronegative or electropositive in nature gets a partial positive charge.
Answer to Problem 93P
Polar.
Explanation of Solution
The structure of caffeine is as follows:
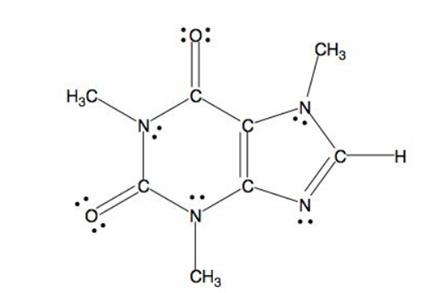
The bond formed between electronegative and electropositive atom will be polar in nature. Here, nitrogen and oxygen atoms are two electronegative atoms and carbon is electropositive atom. Thus, the caffeine molecule is polar in nature.
(i)
Interpretation:
The balanced chemical reaction for combustion of caffeine should be determined.
Concept Introduction:
The caffeine is a chemical, which is found abundantly in some beverage like tea, coffee, cola and mate etc. It is known to be a nervous system stimulant and used to improve the alertness of the brain.
From mass and molar mass, number of moles of substance can be calculated as follows:
Here, n is number of moles, m is mass and m is molar mass.
Answer to Problem 93P
Explanation of Solution
The molecular formula of caffeine is
The combustion reaction takes place in the presence of oxygen. It is given that combustion of caffeine gives nitrogen dioxide. Combustion also produces carbon dioxide and water thus, the complete reaction for combustion of caffeine will be:
To balance the chemical reaction, give coefficient 2 to
(j)
Interpretation:
The heat of combustion for caffeine is 2211 kcal/mol. The heat given off if 0.81 g of caffeine is burned should be calculated.
Concept Introduction:
The caffeine is a chemical, which is found abundantly in some beverage like tea, coffee, cola and mate etc. It is known to be a nervous system stimulant and used to improve the alertness of the brain.
From mass and molar mass, number of moles of substance can be calculated as follows:
Here, n is number of moles, m is mass and m is molar mass.
Answer to Problem 93P
9.22 kcal.
Explanation of Solution
The heat of combustion for caffeine is 2211 kcal/mol. This means 2211 kcal of heat is released in combustion of 1 mol of caffeine. The mass of caffeine is 0.81 g and its molar mass is 194.2 g/mol thus, number of moles can be calculated as follows:
Putting the values,
Since,
Thus,
Therefore, heat of combustion is 9.22 kcal.
(k)
Interpretation:
The weight of
Concept Introduction:
The caffeine is a chemical, which is found abundantly in some beverage like tea, coffee, cola and mate etc. It is known to be a nervous system stimulant and used to improve the alertness of the brain.
From mass and molar mass, number of moles of substance can be calculated as follows:
Here, n is number of moles, m is mass and m is molar mass.
Answer to Problem 93P
3.708 g.
Explanation of Solution
The balanced chemical equation for combustion of caffeine is as follows:
The number of moles of caffeine can be calculated as follows:
Putting the values,
Similarly, number of moles of oxygen gas will be:
From the balanced chemical reaction, 2 moles of caffeine reacts with 27 moles of oxygen thus, 1 mol reacts with 13.5 mol of oxygen.
Thus, 0.0412 mol of caffeine required
Now, from balanced chemical reaction, 2 moles of caffeine gives 10 moles of
Thus, number of moles of
Putting the values,
Thus, the weight of
Want to see more full solutions like this?
Chapter 4 Solutions
Introduction to General, Organic and Biochemistry
- N Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. NH O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic O nonaromatic Garrow_forwardThe conjugate base of alkanes is called alkides. Correct?.arrow_forwardName these organic compounds: structure Br name CH3 CH3 ☐ ☐arrow_forward
- HH H-C H -C-H HH Draw the Skeletal Structures & H Name the molecules HH H H H H-C-C-C-C-C-C-H HHH HHH H H HHHHHHH H-C-C-C-C-C-C-C-C-C-H HHHHH H H H Harrow_forwarddont provide AI solution .... otherwise i will give you dislikearrow_forwardName these organic compounds: structure name CH3 CH3 ☐ F F CH3 ☐ O Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms ofarrow_forward
- Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. ZI NH Explanation Check O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic H O nonaromatic O aromatic O antiaromatic O nonaromatic ×arrow_forwardPart I. Draw the stepwise reaction mechanism of each product (a, b, c, d, e, f) HO HO OH НОН,С HO OH Sucrose HO CH₂OH H N N HO -H H -OH KMnO4, Heat H OH CH₂OH (d) Phenyl Osatriazole OH НОН,С HO HO + Glacial HOAC HO- HO CH₂OH OH HO Fructose (a) Glucose OH (b) H₂N HN (c) CuSO4-5H2O, ethanol H N N N HO ·H H OH H OH N CH₂OH OH (f) Phenyl Osazone H (e) Carboxy phenyl osatriazole Figure 2.1. Reaction Scheme for the Total Synthesis of Fine Chemicalsarrow_forwardWhich molecule is the most stable? Please explain.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





