
CNCT ORG CHEM 6 2020
6th Edition
ISBN: 9781266807244
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 68P
Hydrocarbons like benzene are
phenols. This is an example of a general process in the body, in which an unwanted compound
(benzene) is converted to a more water-soluble derivative called a metabolite, so that it can be
excreted more readily from the body.
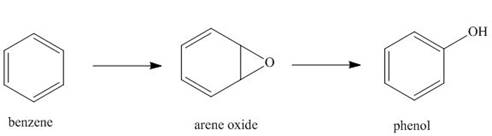
a. Classify each of these reactions as oxidation, reduction, or neither.
b. Explain why phenol is more water soluble than benzene. This means that phenol dissolves in urine, which is largely water, to a greater extent than benzene.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the virtual orbitals for the planar and pyramidal forms of CH3 and for the linear and bent forms of CH2
Q2: Draw the molecules based on the provided nomenclatures below:
(2R,3S)-2-chloro-3-methylpentane:
(2S, 2R)-2-hydroxyl-3,6-dimethylheptane:
Q3: Describes the relationship (identical, constitutional isomers, enantiomers or diastereomers)
of each pair of compounds below.
ག
H
CH3
OH
OH
CH3
H3C
OH
OH
OH
//////////
C
CH3
CH3
CH3
CH3
H3C
CH 3
C/III.....
Physics & Astronomy
www.physics.northweste
COOH
H
нош.....
H
2
OH
HO
CH3
HOOC
H
CH3
CH3
CH3
Br.
H
H
Br
and
H
H
H
H
Chapter 4 Solutions
CNCT ORG CHEM 6 2020
Ch. 4.1 - Prob. 1PCh. 4.1 - Problem 4.2 Which of the following is not another...Ch. 4.1 - Problem 4.3 Draw the five constitutional isomers...Ch. 4.1 - Prob. 4PCh. 4.1 - Prob. 5PCh. 4.2 - Draw the five constitutional isomers that have...Ch. 4.4 - Problem 4.7 Give the IUPAC name for each...Ch. 4.4 - Give the IUPAC name for each compound. a....Ch. 4.4 - Problem 4.9 Give the structure corresponding to...Ch. 4.4 - Prob. 10P
Ch. 4.5 - Give the IUPAC name for each compound.Ch. 4.5 - Give the structure corresponding to each IUPAC...Ch. 4.8 - Arrange the following compounds in order of...Ch. 4.9 - Problem 4.14 Draw the staggered and eclipsed...Ch. 4.9 - Prob. 15PCh. 4.9 - Prob. 16PCh. 4.10 - Problem 4.17 a. Draw the three staggered and...Ch. 4.10 - Problem 4.18 Rank the following conformations in...Ch. 4.10 - Problem 4.19 Consider rotation around the...Ch. 4.10 - Calculate the destabilization present in each...Ch. 4.12 - Problem 4.21 Classify the ring carbons as up or...Ch. 4.12 - Problem 4.22 Using the cyclohexane with the C’s...Ch. 4.13 - Draw a second chair conformation for each...Ch. 4.13 - Problem 4.24 Draw both conformations for and...Ch. 4.13 - Problem 4.25 Draw the structure for each compound...Ch. 4.13 - Prob. 26PCh. 4.14 - Prob. 31PCh. 4.14 - Prob. 32PCh. 4.15 - Prob. 33PCh. 4 - Name each alkane using the ball-and-stick model,...Ch. 4 -
4.40 Draw the structure corresponding to each...Ch. 4 - 4.42 Give the IUPAC name for each compound.
a....Ch. 4 - Prob. 42PCh. 4 - 4.46 Considering rotation around the bond...Ch. 4 - 4.50 Calculate the barrier to rotation for each...Ch. 4 - 4.51 The eclipsed conformation of is less...Ch. 4 - (a) Draw the anti and gauche conformations for...Ch. 4 - For each compound drawn below: a.Label each OH,Br...Ch. 4 - Draw the two possible chair conformations for...Ch. 4 - For each compound drawn below: a. Draw...Ch. 4 - Classify each pair of compounds as constitutional...Ch. 4 - Prob. 66PCh. 4 - 4.64 Draw the products of combustion of each...Ch. 4 - 4.65 Hydrocarbons like benzene are metabolized in...Ch. 4 - Prob. 69PCh. 4 - Prob. 70PCh. 4 - Cyclopropane and cyclobutane have similar strain...Ch. 4 - Prob. 72PCh. 4 - Haloethanes (CH3CH2X,X=Cl,Br,I) have similar...Ch. 4 - Prob. 74PCh. 4 - Prob. 75PCh. 4 - Consider the tricyclic structure B (a) Label each...Ch. 4 - Read Appendix B on naming branched alkyl...Ch. 4 - Read Appendix B on naming bicyclic compounds. Then...
Additional Science Textbook Solutions
Find more solutions based on key concepts
To test your knowledge, discuss the following topics with a study partner or in writing ideally from memory. Th...
HUMAN ANATOMY
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q1: For each molecule, assign each stereocenter as R or S. Circle the meso compounds. Label each compound as chiral or achiral. OH HO CI Br H CI CI Br CI CI Xf x f g Br D OH Br Br H₂N R. IN Ill I -N S OMe D II H CO₂H 1/111 DuckDuckGarrow_forwardThese are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic". All of the carbon atoms of the products must come from the starting material! ? H Harrow_forwardQ5: Draw every stereoisomer for 1-bromo-2-chloro-1,2-difluorocyclopentane. Clearly show stereochemistry by drawing the wedge-and-dashed bonds. Describe the relationship between each pair of the stereoisomers you have drawn.arrow_forward
- Classify each pair of molecules according to whether or not they can participate in hydrogen bonding with one another. Participate in hydrogen bonding CH3COCH3 and CH3COCH2CH3 H2O and (CH3CH2)2CO CH3COCH3 and CH₂ CHO Answer Bank Do not participate in hydrogen bonding CH3CH2OH and HCHO CH3COCH2CH3 and CH3OHarrow_forwardNonearrow_forwardQ4: Comparing (3S,4S)-3,4-dimethylhexane and (3R,4S)-3,4-dimethylhexane, which one is optically active? Briefly explain.arrow_forward
- Nonearrow_forwardNonearrow_forwardGiven the standard enthalpies of formation for the following substances, determine the reaction enthalpy for the following reaction. 4A (g) + 2B (g) → 2C (g) + 7D (g) AHrxn =?kJ Substance AH in kJ/mol A (g) - 20.42 B (g) + 32.18 C (g) - 72.51 D (g) - 17.87arrow_forward
- Determine ASran for Zn(s) + 2HCl(aq) = ZnCl2(aq) + H2(aq) given the following information: Standard Entropy Values of Various Substance Substance So (J/mol • K) 60.9 Zn(s) HCl(aq) 56.5 130.58 H2(g) Zn2+(aq) -106.5 55.10 CI (aq)arrow_forward3) Catalytic hydrogenation of the compound below produced the expected product. However, a byproduct with molecular formula C10H12O is also formed in small quantities. What is the by product?arrow_forwardWhat is the ΔHorxn of the reaction? NaOH(aq) + HCl(aq) → H2O(l) + NaCl(aq) ΔHorxn 1= ________ kJ/molarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY