
ORGANIC CHEMISTRY (LL)-PACKAGE
8th Edition
ISBN: 9781319316389
Author: VOLLHARDT
Publisher: MAC HIGHER
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4, Problem 57P
Interpretation Introduction
Interpretation:Cycloalkane that has the greatest ring strain should be chosen.
Concept introduction:Ring strainis a kind of instability that results when bonds deviate from ideal bond-angle. The cyclic system has different amount of strain as illustrated below:
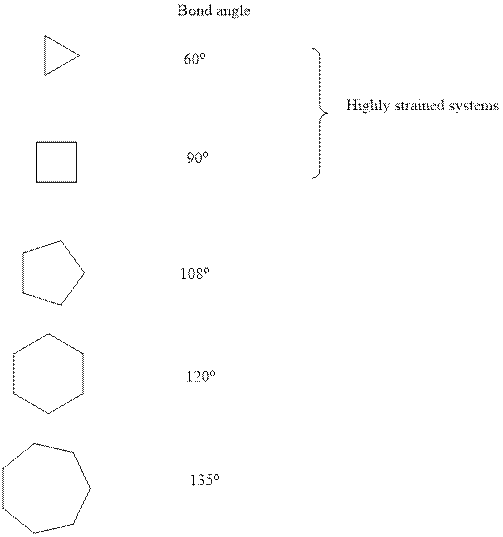
It is evident that three and four membered small ring systems have considerable degree of angular and ring strain that leads to their instability.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Concentration (mg/l)
Peak Area
0
158
10
10241
20
18425
30
26457
40
37125
50
44256
60
56124
Question: Determine the regression equation (a and b coefficients) from first principles
Concentration (mg/l)
Peak Area
0
158
10
10241
20
18425
30
26457
40
37125
50
44256
60
56124
You have been asked to determine the concentration of citral in a highly valued magnolia essential oil.
QUESTION: Calculate the concentration of citral in your highly valued magnolia essential oil which returns a peak area of 41658
Need help with these problems...if you can please help me understand problems E & F.
Chapter 4 Solutions
ORGANIC CHEMISTRY (LL)-PACKAGE
Ch. 4.1 - Prob. 4.1ECh. 4.1 - Prob. 4.3TIYCh. 4.2 - Prob. 4.4ECh. 4.2 - Prob. 4.6TIYCh. 4.3 - Prob. 4.7ECh. 4.4 - Prob. 4.8ECh. 4.4 - Prob. 4.10TIYCh. 4.4 - Prob. 4.11ECh. 4.4 - Prob. 4.12ECh. 4.4 - Prob. 4.14TIY
Ch. 4.6 - Prob. 4.15ECh. 4.7 - Prob. 4.16ECh. 4.7 - Prob. 4.17ECh. 4.7 - Prob. 4.18ECh. 4 - Prob. 21PCh. 4 - Prob. 22PCh. 4 - Prob. 23PCh. 4 - Prob. 24PCh. 4 - Prob. 25PCh. 4 - Prob. 26PCh. 4 - Prob. 27PCh. 4 - Prob. 28PCh. 4 - Prob. 29PCh. 4 - Prob. 30PCh. 4 - Prob. 31PCh. 4 - Prob. 32PCh. 4 - Prob. 33PCh. 4 - Prob. 34PCh. 4 - Prob. 35PCh. 4 - Prob. 36PCh. 4 - Prob. 37PCh. 4 - Prob. 38PCh. 4 - Prob. 39PCh. 4 - Prob. 40PCh. 4 - Prob. 41PCh. 4 - Prob. 42PCh. 4 - Prob. 43PCh. 4 - Prob. 44PCh. 4 - Prob. 45PCh. 4 - Prob. 46PCh. 4 - Prob. 47PCh. 4 - Prob. 48PCh. 4 - Prob. 49PCh. 4 - Prob. 50PCh. 4 - Prob. 51PCh. 4 - Prob. 52PCh. 4 - Prob. 53PCh. 4 - Prob. 54PCh. 4 - Prob. 55PCh. 4 - Prob. 56PCh. 4 - Prob. 57PCh. 4 - Prob. 58PCh. 4 - Prob. 59PCh. 4 - Prob. 60P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me solve these problems. Thank you in advance.arrow_forwardPredict the products of this organic reaction: O N IN A N + H2O + HCI ? Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. 田 C + Explanation Check Click and drag to start drawing a structure. C © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forward6. For each of the following, fill in the synthesis arrows with reagents and show the intermediates. You DO NOT need to use the same number of arrows that are shown (you may use more or less), but the product must be formed from the reactant. Then write the mechanism of one step in the synthesis (you can choose which step to write the mechanism for), including all reagents required, clearly labeling the nucleophile and electrophile for each step, and using curved arrows to show the steps in the mechanism. a. b. OHarrow_forward
- Draw the productsarrow_forwardDraw the correct productsarrow_forwardE Organic Chemistry Maxwell Draw the correct products, in either order, for the ozonolysis reaction: 1) O3, CH2Cl2, -78 °C Product 1 + Product 2 2) Zn, HOAc Draw product 1. Select Draw Templates More C H O presented by M Draw product 2. Erase Select Draw Templates M / # # carrow_forward
- ✓ edict the products of this organic reaction: ---- ။ A CH3–C−NH–CH2–C−CH3 + KOH ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibiliarrow_forwardPredict the product of this organic reaction: A HO-C-CH3 + CH3NH2 P+ H2O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. Explanation Check Click anywhere to draw the first atom of your structure. marrow_forwardH 1) OsO4, pyridine 2) Na2SO3 or NaHSO3 in H₂O 2 productsarrow_forward
- ● Biological Macromolecules Naming and drawing cyclic monosaccharides Your answer is incorrect. • Row 1: Your answer is incorrect. Row 3: Your answer is incorrect. • Row 4: Your answer is incorrect. Try again... 0/5 Give the complete common name, including anomer and stereochemistry labels, of the following molecules. You will find helpful information in the ALEKS resource. CH2OH OH OH H H I H OH OH H] H CH2OH H OH ẞ-L-sorbose HOCH2 OH OH H HOCH2 H OH OH H OH H H CH2OH OH H H OH H I- H OH H OH Explanation Recheck W E R % 25 α B Y X & 5 D F G H McGraw Hill LLC. All Rights Reserved. Terms of Use | Pr Parrow_forwardWhat is the missing reactant in this organic reaction? + R -A HO IN + H₂O Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note for advanced students: you may assume no products other than those shown above are formed. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardStuc X ctclix ALE X A ALE אן A ALEX Lab (195 X Nut x M Inb x NU X NUT X Unt x + → C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IQ1g8NUi-mObKa_ZLx2twjEhK7mVG6PulJI006NcKTV37JxMpZuyrVCdQolLAKqp_7U3r1GUD3... New Chrome available: Naomi Question 26 of 39 (4 points) | Question Attempt: 1 of Unlimited Give the IUPAC name. 2,3-dimethylhexane Part: 1/2 Part 2 of 2 Draw the skeletal structure of a constitutional isomer of the alkane above that contains a different number of carbons in its longest chain. Skip Part Check Click and drag to start drawing a structure. 3 Finance headline Q Search mwa Harvard Intensifi... X Save For Later 00 dlo HB Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility a 9:11 PM 4/22/2025arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning