
Identify the atomic ground-state electron configurations that do not exist. For those that do exist, identify the element.
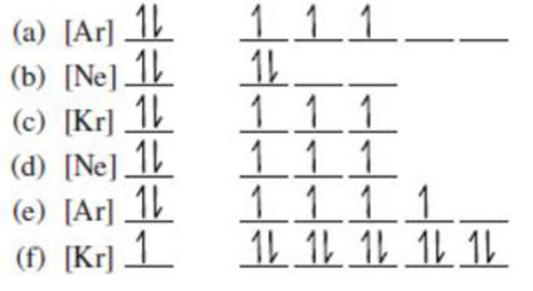
(a)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration exist for (a) and the element is identified as vanadium.
Explanation of Solution
Ground-state electronic configuration of the given element (a) is,
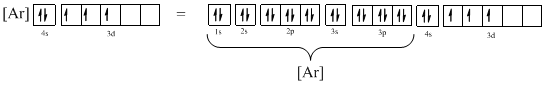
The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete argon configuration along with two electrons in 4s orbital and three electrons in 3d subshell. This is derived as shown above. As the 3d subshell is filled singly, Hund’s rule is followed. Hence this atomic ground-state configuration exists.
Element with the given atomic ground-state configuration is
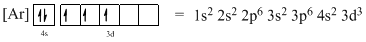
Argon is a noble gas and has a complete octet electronic configuration as
(b)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration does not exist for (b).
Explanation of Solution
Ground-state electronic configuration of (b)
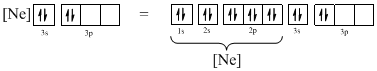
The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete neon configuration along with two electrons in 3s subshell and two electrons in 3p subshell. This is derived as shown above. From the above drawn configuration we can find that the all the orbitals in 3p subshell is not singly filled before pairing. As this does not comply with Hund’s rule, the given atomic ground-state configuration does not exist.
(c)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration does not exist for (c).
Explanation of Solution
Ground-state electronic configuration of the given element (c)

The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete krypton configuration along with two electrons in “s” subshell and three electrons in “p” subshell. This is derived as shown above. From the above drawn configuration we can find that the filling of orbitals does not take place according to the increasing order of energy. After filling of 5s orbital, 4d orbital has to be filled first before 5p orbital is getting filled. But in this case 5p subshell is filled with electrons. It does not comply with Hund’s rule and hence the given atomic ground-state configuration does not exist.
(d)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration exists for (d) and the element is identified as phosphorous.
Explanation of Solution
Ground-state electronic configuration of the given element (d) is,

The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete argon configuration along with two electrons in 4s orbital and three electrons in 3d orbital. This is derived as shown above. As the 3d subshell is filled singly, Hund’s rule is followed. Hence this atomic ground-state configuration exists.
Element with the given atomic ground-state configuration

Neon is a noble gas and has a complete octet electronic configuration as
(e)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration does not exist for (e).
Explanation of Solution
Electronic configuration of given element (e) is,
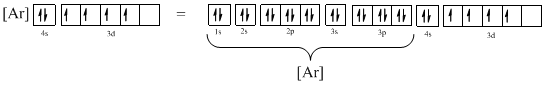
The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete argon configuration along with two electrons in 4s orbital and four electrons in 3d subshell. This is derived as shown above. As the 3d subshell is filled singly, Hund’s rule is followed. Hence this configuration exists.
Atomic ground-state configuration
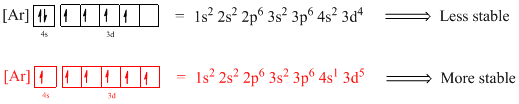
Argon is a noble gas and has a complete octet electronic configuration as
(f)
Interpretation: The atomic ground-state configuration that do not exist for the given sets need to be identified and the elements for atomic ground-state configuration that exists need to be identified.
Concept Introduction:
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. For atoms and ions the electronic configuration are written by using Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbitals is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin. The electrons are filled from lowest energy orbital first.
- Half-filled orbitals are comparatively stable as completely filled orbitals. Therefore if there is a possibility of forming half filled orbital then the electron will be moved to the respective orbitals giving rise to more stability instead of being incompletely filled orbital.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The given atomic ground-state configuration that do not exist and if it exists the element with the atomic ground-state configuration to be identified.
Answer to Problem 4.84QP
Answer
Atomic ground-state configuration exists for (f) and the element is identified as silver.
Explanation of Solution
Ground-state electronic configuration of given element (f) is,

The atomic ground-state configuration is given in the problem statement. From this we can find that this has a complete Krypton configuration along with one electrons in 5s orbital and ten electrons in 4d subshell. This is derived as shown above. As the filling of subshells follows the Hund’s rule, the given atomic ground-state configuration exist.
Element with the given atomic ground-state configuration
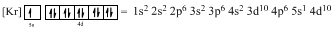
Argon is a noble gas and has a complete octet electronic configuration as
Want to see more full solutions like this?
Chapter 4 Solutions
CHEMISTRY:ATOMS FIRST (LL)>CUSTOM PKG.<
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





