
Concept explainers
Rank the following ions in order of increasing number of unpaired electrons: Tr3+, Fe2+, V3+, Cu+, Mn4+.
Interpretation: It should be ranked the given set of ions in increasing order of number of unpaired electrons.
Concept Introduction:
- Aufbau Principle tells that the orbital with the lower energy is filled with electrons first and then the filling of higher energy orbital follows. By using this, valence orbital diagram can be draw for any atoms or ions.
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. The electronic configurations are writing for atoms and ions, in accordance with Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbital is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin.
To determine the increasing order of unpaired electrons present in the given set of ions.
Answer to Problem 4.79QP
Ions in increasing order of unpaired electrons,
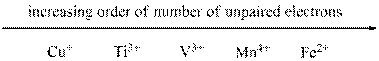
Explanation of Solution
Electronic configuration of
The valence orbital diagram of
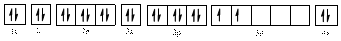
The electronic configuration of
Electronic configuration of
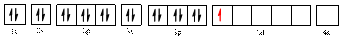
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there is one unpaired electron and which is highlighted in red colour.
Electronic configuration of
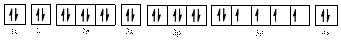
The electronic configuration of
Electronic configuration of
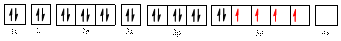
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there are four unpaired electrons that are highlighted in red colour.
Electronic configuration of
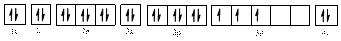
The electronic configuration of
Electronic configuration of
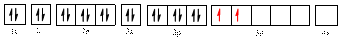
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there are two unpaired electrons, that are highlighted in red colour.
Electronic configuration of
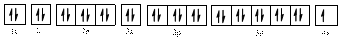
The electronic configuration of
Electronic configuration of
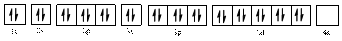
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this we can find that there are no unpaired electrons present.
Electronic configuration of
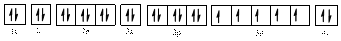
The electronic configuration of
Electronic configuration of
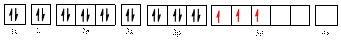
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there are three unpaired electrons present and the same is highlighted in red colour.
Given ions were ranked in increasing order of unpaired electron as follows,
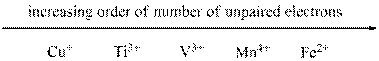
From the number of unpaired electrons for each of the given ions found out in the previous steps we can arrange them in increasing order of number of unpaired electrons as shown above.
The given sets of ions were ranked in increasing order of number of unpaired electrons.
Want to see more full solutions like this?
Chapter 4 Solutions
CHEMISTRY:ATOMS FIRST (LL)>CUSTOM PKG.<
- Explain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forward
- Indicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forwardFor the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forward
- Name the following molecules using iupacarrow_forwardWrite the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





