
Interpretation:
The value of
Concept introduction:
The Gibb’s free energy of mixing determines whether the process of mixing is spontaneous or not at a constant pressure and temperature. The expression to calculate the Gibb’s free energy of mixing is shown below.
Answer to Problem 4.76E
The graph between
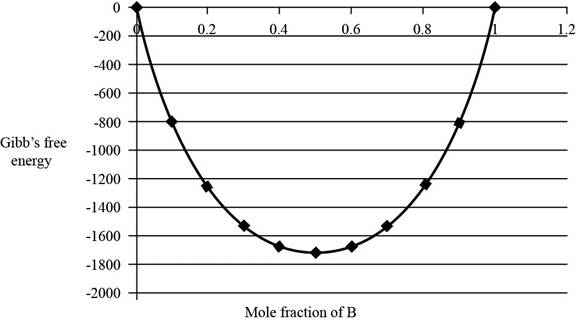
The value of
Explanation of Solution
The formula to calculate Gibb’s free energy of mixing is shown below.-
Where,
•
•
•
•
•
Conversion of temperature from Celsius to Kelvin is shown below.
Substitute the temperature
The temperature is
The mole fraction of gas A and gas B is equal to one as shown below.
Substitute the value of
The value of
Substitute the values of
The value of
Similarly, the value of
Therefore, the value of
The graph between
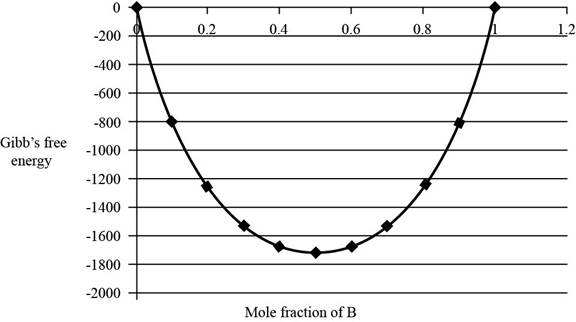
Figure 1
The graph between
Want to see more full solutions like this?
Chapter 4 Solutions
Student Solutions Manual for Ball's Physical Chemistry, 2nd
- The reaction Q(g) + R(g) → Z(l) is shown to be exothermic. Which of the following is true concerning the reactionarrow_forwardWhich of the following has the largest standard molar entropy, S° (298.15 K) He H2 NaCl KBr Hgarrow_forwardWhich of the following is true for a particular reaction if ∆G° is -40.0 kJ/mol at 290 K and –20.0 kJ/mol at 390 K?arrow_forward
- Choose the major product of the reaction with correct regio- and stereochemistry. Br2 H₂O O "Br Br & O 'Br OH Br 吡 O OH OH Br "OH Brarrow_forwardSelect the major product of the following reaction. & Br (CH)CONa (CH₂),COH 0 OC(CH) O &arrow_forwardDraw the products of the hydrolysis reaction between the ester molecule and water. Determine the products of the following reaction.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





