
Concept explainers
(a)
Interpretation:
The IUPAC name of the given structure is to be stated.
Concept introduction:
The systematic naming of organic compound is given by
Rules for writing IUPAC name from the structural formula are given below.
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents is present.
• Name the substituents in alphabetical order.
Answer to Problem 4.45AP
The IUPAC name of the structure is written as
Explanation of Solution
The parent chain of the given structure is numbered as shown below.
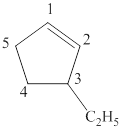
Figure 1
In this structure, total five carbon atoms are present in a cyclic ring which suggest the name to be cyclopentane. It contains a double bond between
The IUPAC name of the given structure is written as
(b)
Interpretation:
The IUPAC name of the given structure is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from the structural formula are given below.
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents is present.
• Name the substituents in alphabetical order.
Answer to Problem 4.45AP
The IUPAC name of the given structure is written as
Explanation of Solution
The parent chain of the given structure is numbered as shown below.
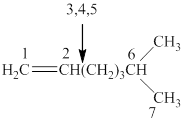
Figure 2
In this structure, total seven carbon atoms are present in a parent chain which suggest the name to be heptane. It also contains a double bond at locant position
The IUPAC name of the given structure is named as
(c)
Interpretation:
The IUPAC name of the given structure is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from the structural formula are given below.
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents is present.
• Name the substituents in alphabetical order.
Answer to Problem 4.45AP
The IUPAC name of the structure is
Explanation of Solution
The parent chain of the given structure is numbered as shown below.
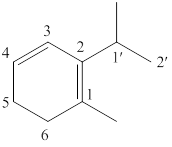
Figure 3
In this structure, total six carbon atoms are present in the cyclic ring which suggest the name to be cyclohexane. It also contained two double bonds at locant positions
The IUPAC name of the given structure is named as shown below.
(d)
Interpretation:
The IUPAC name of the given structure is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from the structural formula are given below.
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents is present.
• Name the substituents in alphabetical order.
Answer to Problem 4.45AP
The IUPAC name of the given structure is written as
Explanation of Solution
The parent chain of the given structure is numbered as shown below.
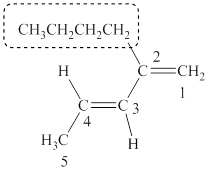
Figure 4
In this structure, total five cabon atoms are presnt in a chain which suggest the name to be pentane. It also conatins two double bonds at locant position
The IUPAC name of the given structure is named as
(e)
Interpretation:
The IUPAC name of the given structure is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from the structural formula are given below.
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents is present.
• Name the substituents in alphabetical order.
Answer to Problem 4.45AP
The IUPAC name of the given structure is written as
Explanation of Solution
The parent chain of the given structure is numbered as shown below.
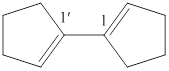
Figure 5
This molecule contains two similar cyclic ring structure means bi is used in this case. In this structure, total five carbon atoms are present with a double bond at position
The IUPAC name of the given structure is named as
(f)
Interpretation:
The IUPAC name of the given structure is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from the structural formula are given below.
• First, identify the longest carbon chain.
• The next step is to identify the groups attached to the longest chain.
• Identify the position, location, and a number of the substituents bonded to the carbon chain.
• Use prefix di, tri, tetra if the same type of substituents is present.
• Name the substituents in alphabetical order.
Answer to Problem 4.45AP
The IUPAC name of the given structure is written as shown below.
Explanation of Solution
The parent chain of the given structure is numbered as shown below.
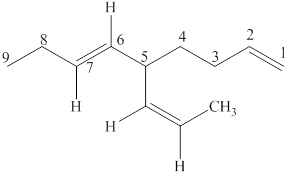
Figure 6
In this structure, total nine carbon atoms are present which suggest the name to be nonane. It also contains two double bonds in the parent chain at positions
The IUPAC name of the given structre is named as shown below.
Want to see more full solutions like this?
Chapter 4 Solutions
ORGANIC CHEM +SG +SAPLING >IP<
- 3. Synthesize the following synthon from the indicated starting material. i HO.arrow_forwardIdentifying the stereochemistry of natural Write the complete common (not IUPAC) name of each molecule below. Note: if a molecule is one of a pair of enantiomers, be sure you start its name with D- or L- so we know which enantiomer it is. molecule H O-C-CH2 H3N. HN N H C=O common name (not the IUPAC name) NH3 ☐ H3N H ☐ CH2 Xarrow_forward> Draw the structure of alanine at pH 1.2. Click and drag to start drawing a structure.arrow_forward
- Understanding the general acid-base properties of amino acids O Proteins Imagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule The solution is... 010 H3N-CH-C-OH CH HO CH3 O acidic O basic neutral O (unknown) H3N HO 0 O acidic O basic neutral ○ (unknown) H3N-CH-C-O CH2 CH3-CH-CH3 O acidic O basic Oneutral ○ (unknown) O= X H2N-CH-C-O CH3 CH CH3 acidic O basic O neutral ○ (unknown) ? 000arrow_forwardImagine each of the molecules shown below was found in an aqueous solution. Can you tell whether the solution is acidic, basic, or neutral? molecule 0=0 H3N-CH-C-o HO CH2 OH The solution is... O acidic O basic O neutral O (unknown) H₂N acidic O basic O neutral ○ (unknown) + H3N O OH O acidic O basic O neutral O (unknown) H2N-CH-C-O CH3 O acidic O basic neutral ○ (unknown) X ? olo HEarrow_forwardRecognizing ampli Draw an a amino acid with a methyl (-CH3) side chain. Explanation Check Click and drag to start drawing a structure. X Carrow_forward
- Write the systematic name of each organic molecule: structure name × HO OH ☐ OH CI CI O CI OH OHarrow_forwardく Check the box under each a amino acid. If there are no a amino acids at all, check the "none of them" box under the table. Note for advanced students: don't assume every amino acid shown must be found in nature. COO H3N-C-H CH2 HO CH3 NH3 O CH3-CH CH2 OH Onone of them Explanation Check + H3N O 0. O OH + NH3 CH2 CH3-CH H2N C-COOH H O HIC + C=O H3N-C-O CH3- - CH CH2 OH Х 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accesarrow_forwardWrite the systematic name of each organic molecule: structure HO-C-CH2-CH3 O -OH CH3-CH2-CH2-CH2-CH2-C-OH CH3 CH3-CH-CH2-C-OH Explanation Check S namearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





