
Concept explainers
(a)
Interpretation:
The Lewis structure for an aldetetrose is to be drawn.
Concept introduction:
Monosaccharides are called simple sugars, with the number of oxygen atoms same as the number of carbon atoms. The general formula for monosaccharides is
Answer to Problem 4.25P
The Lewis structure for an aldetetrose is
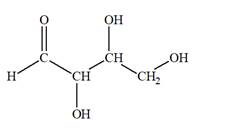
Explanation of Solution
Aldotetrose is a mono-saccharide. The name “aldo” indicates that the carbonyl group must be present at the terminal carbon atom, while the word “tetrose” indicates a chain of four carbon atoms. Thus, aldotetrose is a sugar having an
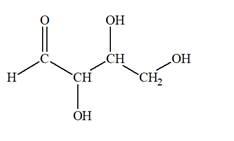
The molecular formula for the above structure is
Altetrose is a mono-saccharide having a carbonyl group at the terminal carbon atom, and its molecular formula is
(b)
Interpretation:
The Lewis structure for a ketotetrose is to be drawn.
Concept introduction:
Monosaccharides are called simple sugars, with the number of oxygen atoms same as the number of carbon atoms. The general formula for a monosaccharide is
Answer to Problem 4.25P
The Lewis structure for a ketotetrose is
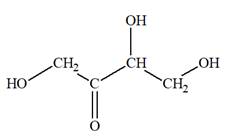
Explanation of Solution
Ketotetrose is a type of mono-saccharide. The name “keto” indicates that the carbonyl group must be present at the internal carbon atom, while the word “tetrose” indicates the chain of four carbon atoms. Thus, ketotetrose is a sugar having a carbonyl group at the internal carbon atom, and the structure must have four carbon atoms. The general molecular formula for mono-saccharides is
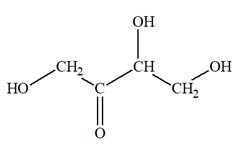
The molecular formula for the above structure is
Ketotetrose is a mono-saccharide having a carbonyl group at the internal carbon atom, and its molecular formula is
(c)
Interpretation:
The Lewis structure for an aldetriose is to be drawn.
Concept introduction:
Monosaccharides are called simple sugars, with the number of oxygen atoms same as the number of carbon atoms. Thus, the general molecular formula for a monosaccharide is
Answer to Problem 4.25P
The Lewis structure for an aldetriose is
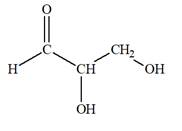
Explanation of Solution
Aldotriose is a type of mono-saccharide. The name “aldo”’ indicates that the carbonyl group must be present at the terminal carbon atom, while the word “triose” indicates a chain of three carbon atoms. Thus, aldotriose is a sugar having an aldehyde group at the terminal carbon atom, and the structure must have three carbon chain. The general molecular formula for mono-saccharides is

The molecular formula for the above structure is
Altetrose is a mono-saccharide having a carbonyl group at the terminal carbon atom, and its molecular formula is
(d)
Interpretation:
The Lewis structure for a ketotriose is to be drawn.
Concept introduction:
Monosaccharides are called simple sugars, with the number of oxygen atoms same as the number of carbon atoms. Thus, the general molecular formula for a monosaccharide is
Answer to Problem 4.25P
The Lewis structure for a ketotriose is
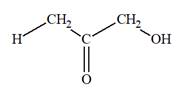
Explanation of Solution
Ketotriose is a type of mono-saccharide. The name “keto” indicates that the carbonyl group must be present at the internal carbon atom, while the word “tetrose” indicates the chain of four carbon atoms. Thus, ketotriose is a sugar having a carbonyl group at the internal carbon atom, and the structure must have three carbon atoms. The general molecular formula for mono-saccharides is

The molecular formula for the above structure is
Ketotetrose is a mono-saccharide having a carbonyl group at the internal carbon atom, and its molecular formula is
(e)
Interpretation:
The Lewis structure for a ketohexose, which is different from fructose, is to be drawn.
Concept introduction:
Monosaccharides are called simple sugars, with the number of oxygen atoms same as the number of carbon atoms. Thus, the general molecular formula for a monosaccharide is
Answer to Problem 4.25P
The Lewis structure for a ketohexose, which is different from fructose, is
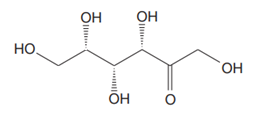
Explanation of Solution
The Lewis structure for fructose is
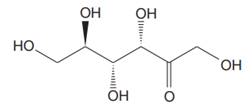
Fructose is a ketohexose with a molecular formula

The molecular formula for the above structure is
Ketohexose is a mono-saccharide having a carbonyl group at the internal carbon atom, and its molecular formula is
Want to see more full solutions like this?
Chapter 4 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- Interpreting NMR spectra is a skill that often requires some amount of practice, which, in turn, necessitates access to a collection of NMR spectra. Beyond Labz Organic Synthesis and Organic Qualitative Analysis have spectral libraries containing over 700 1H NMR spectra. In this assignment, you will take advantage of this by first predicting the NMR spectra for two closely related compounds and then checking your predictions by looking up the actual spectra in the spectra library. After completing this assignment, you may wish to select other compounds for additional practice. 1. Write the IUPAC names for the following two structures: Question 2 Question 3 2. Predict the NMR spectra for each of these two compounds by listing, in the NMR tables below, the chemical shift, the splitting, and the number of hydrogens associated with each predicted peak. Sort the peaks from largest chemical shift to lowest. **Not all slots must be filled**arrow_forward11:14 ... worksheets.beyondlabz.com 3. To check your predictions, click this link for Interpreting NMR Spectra 1. You will see a list of all the - compounds in the spectra library in alphabetical order by IUPAC name. Hovering over a name in the list will show the structure on the chalkboard. The four buttons on the top of the Spectra tab in the tray are used to select the different spectroscopic techniques for the selected compound. Make sure the NMR button has been selected. 4. Scroll through the list of names to find the names for the two compounds you have been given and click on the name to display the NMR spectrum for each. In the NMR tables below, list the chemical shift, the splitting, and the number of hydrogens associated with each peak for each compound. Compare your answers to your predictions. **Not all slots must be filled** Peak Chemical Shift (d) Multiplicity 1 2 3 4 5arrow_forwardО δα HO- H -Br δα HO-- + + -Br [B] 8+ HO- -Br δα नarrow_forward
- 1/2 - 51% + » GAY Organic Reactions Assignment /26 Write the type of reaction that is occurring on the line provided then complete the reaction. Only include the major products and any byproducts (e.g. H₂O) but no minor products. Please use either full structural diagrams or the combination method shown in the lesson. Skeletal/line diagrams will not be accepted. H3C 1. 2. CH3 A Acid OH Type of Reaction: NH Type of Reaction: + H₂O Catalyst + HBr 3. Type of Reaction: H3C 4. Type Reaction: 5. H3C CH2 + H2O OH + [0] CH3 Type of Reaction: 6. OH CH3 HO CH3 + Type of Reaction: 7. Type of Reaction: + [H]arrow_forwardhumbnai Concentration Terms[1].pdf ox + New Home Edit Sign in Comment Convert Page Fill & Sign Protect Tools Batch +WPS A Free Trial Share Inter Concreting Concentration forms. Hydrogen peroxide is a powerful oxidizing agent wed in concentrated solution in rocket fuels and in dilute solution as a hair bleach. An aqueous sulation of H2O2 is 30% by mass and has density of #liligime calculat the Ⓒmolality ⑥mole fraction of molarity. 20 9. B. A sample of Commercial Concentrated hydrochloric ETarrow_forwardIf a reaction occurs, what would be the major products? Please include a detailed explanation as well as a drawing showing how the reaction occurs and what the final product is.arrow_forward
- Would the following organic synthesis occur in one step? Add any missing products, required catalysts, inorganic reagents, and other important conditions. Please include a detailed explanation and drawings showing how the reaction may occur in one step.arrow_forward(a) Sketch the 'H NMR of the following chemical including the approximate chemical shifts, the multiplicity (splitting) of all signals and the integration (b) How many signals would you expect in the 13C NMR? CH3arrow_forwardDraw the Show the major and minor product(s) for the following reaction mechanisms for both reactions and show all resonance structures for any Explain why the major product is favoured? intermediates H-Brarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div





