
Concept explainers
To determine:
The difference between a Lewis structure and a condensed structure in terms of atoms and bonds is shown in the structure.
Introduction:
The molecules of organic compounds are represented by four different ways namely, Lewis structure, skeletal formula, condensed structural formula, and ball-and-stick model.
Lewis structures show all atoms, bonds, and lone pairs of atoms in the molecule. It helps in predicting the molecular geometry and shows the connectivity of atoms in a molecule whereas, condensed structural formula is simpler as it does not show any bonds or lone pairs of atoms and shows no connectivity of atoms in a molecule.
Explanation of Solution
The example of butanol molecule is to be assumed.
The Lewis structure of butanol molecule is represented as,
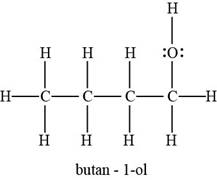
Fig.1
The number of bonds of each atom depends on the valence electrons of atoms in a molecule.
Figure 1 shows the Lewis structure in which carbon has
Condensed structure shows the shorter way to draw a molecule and is more simplified than the Lewis structure. It shows all atoms except the vertical bonds and most or all the horizontal bonds.
The condensed structure of butanol molecule is represented as,
In this formula, first carbon atom has three hydrogen atoms bonded to it; the second, third, and fourth carbon has two hydrogen atoms. The two lone pair on oxygen atoms are not shown as in condensed structure of atoms. Lone pair is not represented in the chemical symbol of atom.
Lewis structure is the most expanded structure as it shows all atoms, bonds, and lone pairs of atoms in a molecule whereas, condensed structure is the least expanded as it does not show all the bonds and lone pairs of atoms. Condensed structure is simpler than the Lewis structure.
Want to see more full solutions like this?
Chapter 4 Solutions
EBK LABORATORY MANUAL FOR GENERAL, ORGA
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
- For the reaction A (g) → 3 B (g), Kp = 0.379 at 298 K. What is the value of ∆G for this reaction at 298 K when the partial pressures of A and B are 5.70 atm and 0.250 atm?arrow_forward14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing 150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3 Ag+ + S20 Ag(S203)¯ K₁ = 7.4 × 108 Ag(S203)¯ + S20¯ = Ag(S203) K₂ = 3.9 x 104arrow_forwardΗΝ, cyclohexanone pH 4-5 Draw Enamine I I CH3CH2Br THF, reflux H3O+ I Drawing Draw Iminium Ionarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning



