
Concept explainers
Interpretation: The velocity component in a particular direction is to be calculated.
Concept introduction: Velocity vector is a sum of the two velocity
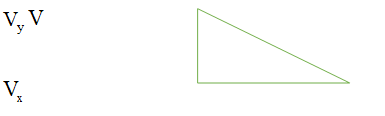
The component in a particular direction is the dot product of the velocity vector and unit vector.
Answer to Problem 4.1P
The component of velocity is 1.66 m/s.
Explanation of Solution
The velocity vector given is,
At point (2,2) the equation (2) can be rewritten as,
A unit vector in
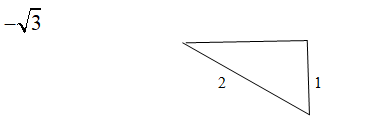
Using equation (1) in the above vector diagram at angle
Taking the dot product of equation (2) and (3) we get,
Thus, the dot product of the velocity vector and the vector in a particular direction gives the velocity component and its value here is 1.66 m/s.
Want to see more full solutions like this?
Chapter 4 Solutions
Fundamentals of Momentum, Heat, and Mass Transfer
- 1) Indicate whether each statement below is True or False: a) In extractions, small proteins usually end up in the heavy phase. b) In extractions, large proteins usually end up in the heavy phase. c) Tie line length on a phase diagram has no effect on protein partitioning. d) For extraction, we assume that each stage reaches equilibrium. e) Isotherms that are concave down lead to self-sharpening solute fronts in adsorption. f) When scaling up an adsorption unit, the LUB scales proportionally with adsorber length. g) For chromatography, separation efficiency increases when HETP increases. h) In size exclusion chromatography, smaller molecules elute from the column first. i) In hydrophobic interaction chromatography, very hydrophobic proteins elute in water. j) Molecules at very high concentrations elute from the column as a perfect Gaussian peak. k) In chromatography scale-up, resolution will increase as column diameter increases. 1) A good adsorbent typically has no porosity. m)…arrow_forwardChemical Engineering Use the psychrometric chart and demonstrate the linear interpolation method to obtain -0.52 KJ/KgDA. This is the enthalpy deviation. The exercise is uploaded below.arrow_forwardChemical Engineering Use the psychrometric chart. The remaining curves on the psychrometric chart are almost vertical and convex to the left, with labeled values (on Figure 8.4-1) of 0.05, 0.1, 0.2, and so on. (The units of these numbers arekJ/kg DA). Thesecurves are usedto determine theenthalpyof humid air that is not saturated. The procedure is as follows: (a) locate the point on the chart corresponding to air at its specified condition; (b) interpolate to estimate the enthalpy deviation at this point; (c) follow the constant wet-bulb temperature line to the enthalpy scale above the saturation curve, read the value on that scale, and add the enthalpy deviation to it. Also, you will see the exercise on the piece of paper.arrow_forward
- Calculate the permeability of the bed of ion-exchange particles in Example 11.1.arrow_forwardchemical engineering problemarrow_forwardA nozzle is attached to a fire hose by a bolted flange as shown below. What is the force tending to tear apart that flange when the valve in the nozzle is closed?arrow_forward
- the answer should be: V2= -(P0-PL/2μL)(dx-x^)+Ux/darrow_forwardFor spherical sand particles with Dp = 0.03 and ρparticles = 150 lbm / ft3 estimate the minimum fluidizing velocity for air and for water. Assume ε = 0.3. In the case of the water we must rederive Eq. 11.42, taking into account the buoyant force on the particles. Below are the provide answers. Please show all work to get to the correct answers.arrow_forwardPlease show all workarrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





