
Concept explainers
(a)
Interpretation:
It is to be determined whether the two given molecules are constitutional isomers of each other.
Concept introduction:
The compounds having same molecular formula but different connectivity of the atoms are constitutional isomers. The arrangement of the atoms is different for constitutional isomers. From the total number of atoms and arrangement of atoms, it is decided that whether the two molecules are constitutional isomers or not.
Answer to Problem 4.16P
The two given molecules are not constitutional isomers of each other.
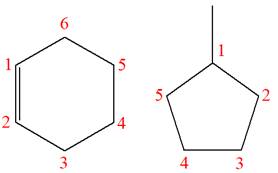
Explanation of Solution
The structures of the given molecules are
The molecular formula of the first compound is
The first molecule contains ten hydrogens, and the second molecule contains twelve hydrogens. However, the total number of hydrogens is different in each molecule. In case of constitutional isomers, the total number of atoms remains the same in the molecular formula. So both molecules are not constitutional isomers of each other.
The constitutional isomers are determined from the molecular formula and connectivity of the atoms.
(b)
Interpretation:
It is to be determined whether the two given molecules are constitutional isomers of each other.
Concept introduction:
The compounds having same molecular formula but different connectivity of the atoms are constitutional isomers. The arrangement of the atoms is different for constitutional isomers. From the total number of atoms and arrangement of atoms, it is decided that whether the two molecules are constitutional isomers or not.
Answer to Problem 4.16P
The two given molecules are not constitutional isomers of each other.
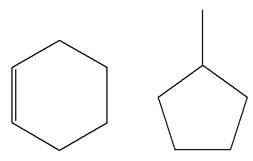
Explanation of Solution
The structures of the given molecules are
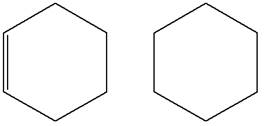
The molecular formula of the first compound is
The first molecule contains ten hydrogens, and the second molecule contains twelve hydrogens. However, the total number of hydrogens is different in each molecule. In case of constitutional isomers, the total number of atoms remains the same in the molecular formula. So both molecules are not constitutional isomers of each other.
The constitutional isomers are determined from the molecular formula and connectivity of the atoms.
(c)
Interpretation:
It is to be determined whether the two given molecules are constitutional isomers of each other.
Concept introduction:
The compounds having same molecular formula but different connectivity of the atoms are constitutional isomers. The arrangement of the atoms is different for constitutional isomers. From the total number of atoms and arrangement of atoms, it is decided that whether the two molecules are constitutional isomers or not.
Answer to Problem 4.16P
The two given molecules are not constitutional isomers of each other.
Explanation of Solution
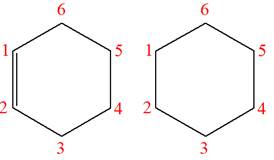
The structures of the given molecules are
The molecular formula of the first compound is

The first molecule contains ten hydrogens, and the second molecule contains twelve hydrogens. However, the total number of hydrogens is different in each molecule. In case of constitutional isomers, the total number of atoms remains the same in the molecular formula. So both molecules are not constitutional isomers of each other.
The constitutional isomers are determined from the molecular formula and connectivity of the atoms.
(d)
Interpretation:
It is to be determined whether the two given molecules are constitutional isomers of each other.
Concept introduction:
The compounds having same molecular formula but different connectivity of the atoms are constitutional isomers. The arrangement of the atoms is different for constitutional isomers. From the total number of atoms and arrangement of atoms, it is decided that whether the two molecules are constitutional isomers or not.
Answer to Problem 4.16P
The two given molecules are constitutional isomers of each other.
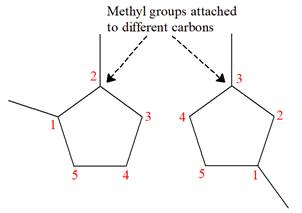
Explanation of Solution
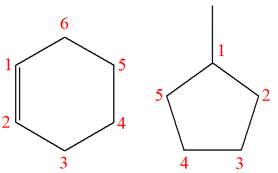
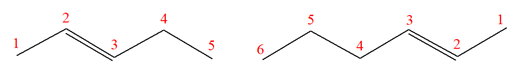
The structures of the given molecules are
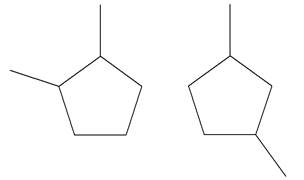
The molecular formula of both molecules is
The number assigned to carbon atoms are same in each molecule based on the first methyl group attached to the ring. However, the connectivity of the methyl group is not the same. In the first molecule, the methyl group is attached to the
The constitutional isomers are determined from the molecular formula and connectivity of the atoms.
(e)
Interpretation:
It is to be determined whether the two given molecules are constitutional isomers of each other.
Concept introduction:
The compounds having same molecular formula but different connectivity of the atoms are constitutional isomers. The arrangement of the atoms is different for constitutional isomers. From the total number of atoms and arrangement of atoms, it is decided that whether the two molecules are constitutional isomers or not.
Answer to Problem 4.16P
The two given molecules are not constitutional isomers of each other.
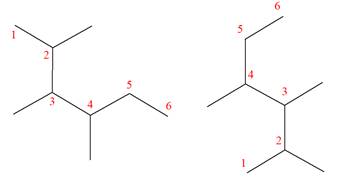
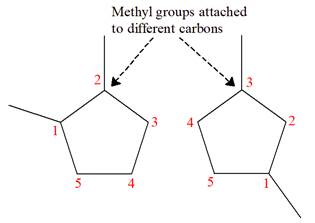
Explanation of Solution
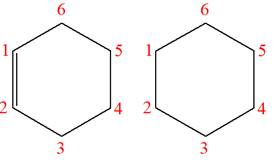
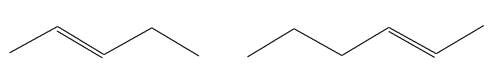
The structures of the given molecules are
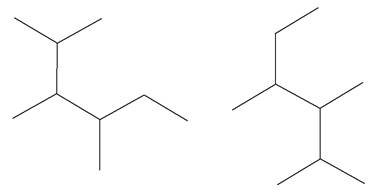
The molecular formula of both molecules is
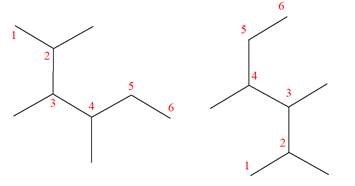
The numbers assigned to carbon atoms are the same in each molecule based on the first methyl group attached to the longest carbon chain. Both molecules have methyl groups attached to the
The constitutional isomers are determined from the molecular formula and connectivity of the atoms.
(f)
Interpretation:
It is to be determined whether the two given molecules are constitutional isomers of each other.
Concept introduction:
The compounds having same molecular formula but different connectivity of the atoms are constitutional isomers. The arrangement of the atoms is different for constitutional isomers. From the total number of atoms and arrangement of atoms, it is decided that whether the two molecules are constitutional isomers or not.
Answer to Problem 4.16P
The two given molecules are not constitutional isomers of each other.
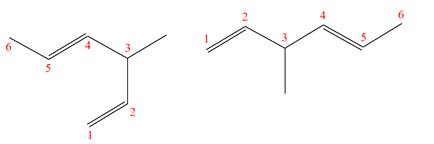
Explanation of Solution
The structures of the given molecules are
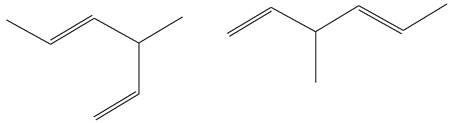
The molecular formula of both molecules is

The numbers assigned to double-bonded carbon atoms are the same in each molecule. In both compounds, the methyl group is connected to
The constitutional isomers are determined from the molecular formula and connectivity of the atoms.
(g)
Interpretation:
It is to be determined whether the two given molecules are constitutional isomers of each other.
Concept introduction:
The compounds having same molecular formula but different connectivity of the atoms are constitutional isomers. The arrangement of the atoms is different for constitutional isomers. From the total number of atoms and arrangement of atoms, it is decided that whether the two molecules are constitutional isomers or not.
Answer to Problem 4.16P
The two given molecules are not constitutional isomers of each other.
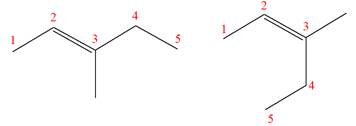
Explanation of Solution
The structures of the given molecules are
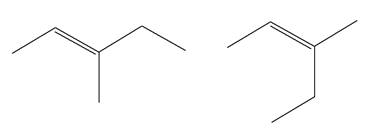
The molecular formula of both molecules is
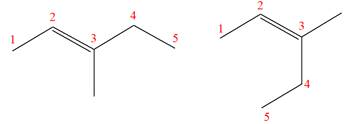
The number assigned to double-bonded carbon atoms are the same in each molecule. In both compounds, the methyl group is connected to
The constitutional isomers are determined from the molecular formula and connectivity of the atoms.
(h)
Interpretation:
It is to be determined whether the two given molecules are constitutional isomers of each other.
Concept introduction:
The compounds having same molecular formula but different connectivity of the atoms are constitutional isomers. The arrangement of the atoms is different for constitutional isomers. From the total number of atoms and arrangement of atoms, it is decided that whether the two molecules are constitutional isomers or not.
Answer to Problem 4.16P
The two given molecules are not constitutional isomers of each other.
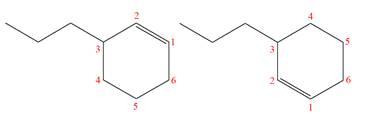
Explanation of Solution
The structures of the given molecules are
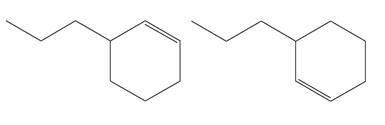
The molecular formula of both molecules is
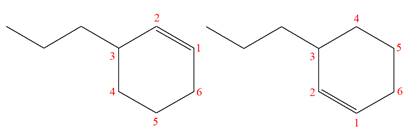
The numbers assigned to double-bonded carbon atoms are the same in each molecule. In both compounds, the propyl group is connected to
The constitutional isomers are determined from the molecular formula and connectivity of the atoms.
(i)
Interpretation:
It is to be determined whether the two given molecules are constitutional isomers of each other.
Concept introduction:
The compounds having same molecular formula but different connectivity of the atoms are constitutional isomers. The arrangement of the atoms is different for constitutional isomers. From the total number of atoms and arrangement of atoms, it is decided that whether the two molecules are constitutional isomers or not.
Answer to Problem 4.16P
The two given molecules are constitutional isomers of each other.
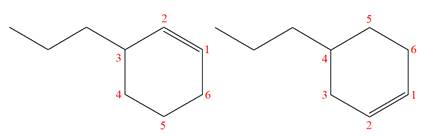
Explanation of Solution
The structures of the given molecules are
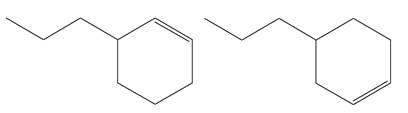
The molecular formula of both molecules is
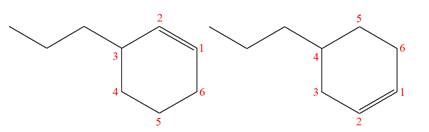
The numbers assigned to double-bonded carbon atoms are the same in each molecule.
However, the connectivity of the propyl group is not the same. In the first molecule, the propyl group is attached to the
The constitutional isomers are determined from the molecular formula and connectivity of the atoms.
Want to see more full solutions like this?
Chapter 4 Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- Predict the major products of the following organic reaction: Some important notes: Δ CN ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. ONO reaction. Click and drag to start drawing a structure.arrow_forwardThe following product was made from diethyl ketone and what other reagent(s)? £ HO 10 2-pentyne 1-butyne and NaNH2 ☐ 1-propanol ☐ pyridine butanal ☐ pentanoatearrow_forwardWhich pair of reagents will form the given product? OH X + Y a. CH3 b. CH2CH3 ༧་་ C. CH3- CH2CH3 d.o6.(རི॰ e. CH3 OCH2CH3 -MgBr f. CH3-MgBr g. CH3CH2-MgBr -C-CH3 CH2CH3arrow_forward
- Question 3 What best describes the product of the following reaction? 1. CH3CH2MgBr (2 eq) 2. H a new stereocenter will not be formed a new stereocenter will be formed an alkyl halide will result an alkane will result an aromatic compound will result 1 ptsarrow_forwardRank the following from most to least reactive toward nucleophilic attack. 1. [Select] [Select] 2. Acyl halide Aldehyde 3. Carboxylate ion 4. Carboxylic acid Ketone 5. [Select]arrow_forwardQuestion 10 1 pts Which of the following is the most accurate nomenclature? 1-hydroxy-1-methyldecane-4,7-dione 2-hydroxy-2-methyldecane-5,8-dione 4,6-dioxo-2-methyldecane-2-ol 9-hydroxy-9-methyldecane-3,6-dione 8-hydroxy-8-methylnonane-3,6-dione OHarrow_forward
- Could you please explain whether my thinking is correct or incorrect regarding how I solved it? Please point out any mistakes in detail, with illustrations if needed.arrow_forwardWhat are the most proper reagents to achieve these products? سد 1. 2. OH ○ 1. BrMgC6H6; 2. H+ ○ 1. BrMgCH2CH2CH2CH2CH3; 2. H+ O 1. CH3CH2CHO; 2. H+ O 1. BrMgCH2CH3; 2. H+arrow_forwardProvide the IUPAC (systematic) name only for the following compound. Dashes, commas, and spaces must be correct. Harrow_forward
- Please use the nernst equation to genereate the Ion Selective Electrode Analysis standard curve within my excel spread sheet. Nernst Equation: E = Eo + m (ln a) Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/EaREe1-PfGNKq1Cbink6kkYB5lBy05hEaE3mbGPUb22S6w?rtime=zQaSX3xY3Ugarrow_forwarda) b) c) H NaOH heat, dehydration + KOH heat, dehydration NaOH + (CH3)3CCHO heat, dehydration Pharrow_forwardshow mechanismarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

