
EBK THE COSMIC PERSPECTIVE
9th Edition
ISBN: 9780135161760
Author: Voit
Publisher: VST
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 4, Problem 3VSC
Use the following questions to check your understanding of some of the many types of visual information used in astronomy. For additional practice, try the Chapter 4 Visual Quiz at MasteringAsrrononry®.
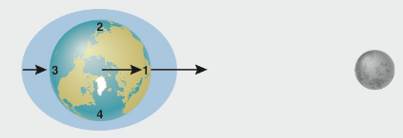
The figure above, based on figure 4.24, shows how the Moon causes tides on Earth. Note that the North Pole is in the center of the diagram, so the numbers 1 through 4 label points along Earth's equator.
3. Where is it low tide?
a. point 1 only
b. point 2 only
c. points 1 and 3
d. points 2 and 4
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. An arrangement of three charges is shown below where q₁ = 1.6 × 10-19 C, q2 = -1.6×10-19 C,
and q3 3.2 x 10-19 C.
2 cm
Y
93
92
91
X
3 cm
(a) Calculate the magnitude and direction of the net force on q₁.
(b) Sketch the direction of the forces on qi
(Figure 1)In each case let w be the weight of the suspended crate full of priceless art objects. The strut is uniform and also has weight w
Find the direction of the force exerted on the strut by the pivot in the arrangement (a).
Express your answer in degrees.
Find the tension Tb in the cable in the arrangement (b).
Express your answer in terms of w.
Find the magnitude of the force exerted on the strut by the pivot in the arrangement (b).
Express your answer in terms of w.
(Figure 1)In each case let ww be the weight of the suspended crate full of priceless art objects. The strut is uniform and also has weight w.
Find the direction of the force exerted on the strut by the pivot in the arrangement (b).
Express your answer in degrees.
Chapter 4 Solutions
EBK THE COSMIC PERSPECTIVE
Ch. 4 - Prob. 1VSCCh. 4 - Use the following questions to check your...Ch. 4 - Use the following questions to check your...Ch. 4 - Use the following questions to check your...Ch. 4 - Use the following questions to check your...Ch. 4 - Define speed, velocity, and acceleration. What are...Ch. 4 - Define momentum and force. What do we mean when we...Ch. 4 - What is free-fall, and why does it make you...Ch. 4 - Prob. 4EAPCh. 4 - Describe the laws of conservation of momentum, of...
Ch. 4 - Define kinetic energy, radiative energy, and...Ch. 4 - Define temperature and thermal energy. How are...Ch. 4 - Prob. 8EAPCh. 4 - 9. Summarize the universal law of gravitation both...Ch. 4 - 10. What is the difference between a bound and an...Ch. 4 - What do we need to know if we want to measure an...Ch. 4 - Explain why orbits cannot change spontaneously,...Ch. 4 - Explain how the Moon creates tides on Earth. Why...Ch. 4 - What is tidal friction? What effects does it have...Ch. 4 - Does It Make Sense?
Decide whether the statement...Ch. 4 - Does It Make Sense?
Decide whether the statement...Ch. 4 - Does It Make Sense?
Decide whether the statement...Ch. 4 - Does It Make Sense?
Decide whether the statement...Ch. 4 - Prob. 19EAPCh. 4 - Prob. 20EAPCh. 4 - Does It Make Sense? Decide whether the statement...Ch. 4 - Does It Make Sense?
Decide whether the statement...Ch. 4 - Does It Make Sense?
Decide whether the statement...Ch. 4 - Does It Make Sense?
Decide whether the statement...Ch. 4 - Choose the best answer to each of the following....Ch. 4 - Choose the best answer to each of the following....Ch. 4 - Choose the best answer to each of the following....Ch. 4 - Choose the best answer to each of the following....Ch. 4 - Choose the best answer to each of the following....Ch. 4 - Prob. 30EAPCh. 4 - Prob. 31EAPCh. 4 - Choose the best answer to each of the following....Ch. 4 - Choose the best answer to each of the following....Ch. 4 - Choose the best answer to each of the following....Ch. 4 - Prob. 35EAPCh. 4 - Testing Gravity. Scientists are continually trying...Ch. 4 - Prob. 38EAPCh. 4 - Prob. 39EAPCh. 4 - Prob. 40EAPCh. 4 - Weightlessness. Astronauts are weightless when in...Ch. 4 - Units of Acceleration. If you drop a rock from a...Ch. 4 - Gravitational Potential Energy. For each of the...Ch. 4 - Prob. 44EAPCh. 4 - The Gravitational Law. How does quadrupling the...Ch. 4 - Allowable Orbits? Suppose the Sun were replaced by...Ch. 4 - Head-to-Foot Tides. You and Earth attract each...Ch. 4 - Prob. 48EAPCh. 4 - Geostationary Orbit. A satellite in geostationary...Ch. 4 - Prob. 51EAPCh. 4 - Prob. 52EAPCh. 4 - Moving Candy Bar. Table 4.1 shows that...Ch. 4 - Spontaneous Human Combustion. Suppose that all the...Ch. 4 - Fusion Power. No one has yet succeeded in creating...Ch. 4 - Understanding Newton’s Version of Kepler’s Third...Ch. 4 - Using Newton’s Version of Kepler’s Third Law....Ch. 4 - Escape Velocity. Calculate the escape velocity...Ch. 4 - Weights on Other Worlds. Calculate the...Ch. 4 - Prob. 60EAPCh. 4 - Extra Moon. Suppose Earth had a second moon,...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A 70.0 cm, uniform, 40.0 N shelf is supported horizontally by two vertical wires attached to the sloping ceiling (Figure 1). A very small 20.0 N tool is placed on the shelf midway between the points where the wires are attached to it. Find the tension in the left-hand wire. Express your answer with the appropriate units.arrow_forwardFind the total bind Mev. binding energy for 13 Carbon, 6C (atomic mass = 13.0033554)arrow_forwardWhat is the 27 energy absorbed in this endothermic Auclear reaction 2] Al + 'n → 27 Mg + ! H? (The atom mass of "Al is 26.981539u. and that of 11 Mg is 26.984341u) MeVarrow_forward
- What is the energy released in this nuclear reaction 1 F + "', H-1 O+ He? 19 19 16 (The atomic mass of 1F is 18.998403 u, and that of 20 is 15.9949154) MeV.arrow_forwardWhat is the energy released in this B+ nuclear reaction خالد 2½ Al w/ Mg + ie? (The atomic mass of 11 Al is 23.9999394 and that > of 12 Mg is 23.985041 u) MeV.arrow_forwardWhat is the energy released / absorbed in this nuclear reaction 14 N+ & He → » O + ! N? (The atomic mass of 14 N is 14.003074u. 17N+ and that of 10 is 16.9991324). MeVarrow_forward
- Can someone help me answer this question thanks.arrow_forwardCan someone help me with this question thanks.arrow_forward4B. Four electrons are located on the corners of a square, one on each corner, with the sides of the square being 25 cm long. a) Draw a sketch of the scenario and use your sketch to b) Determine the total force (magnitude and direction) on one of the electrons from the other three?arrow_forward
- Portfolio Problem 3. A ball is thrown vertically upwards with a speed vo from the floor of a room of height h. It hits the ceiling and then returns to the floor, from which it rebounds, managing just to hit the ceiling a second time. Assume that the coefficient of restitution between the ball and the floor, e, is equal to that between the ball and the ceiling. Compute e.arrow_forwardPortfolio Problem 4. Consider two identical springs, each with natural length and spring constant k, attached to a horizontal frame at distance 2l apart. Their free ends are attached to the same particle of mass m, which is hanging under gravity. Let z denote the vertical displacement of the particle from the hori- zontal frame, so that z < 0 when the particle is below the frame, as shown in the figure. The particle has zero horizontal velocity, so that the motion is one dimensional along z. 000000 0 eeeeee (a) Show that the total force acting on the particle is X F-mg k-2kz 1 (1. l k. (b) Find the potential energy U(x, y, z) of the system such that U x = : 0. = O when (c) The particle is pulled down until the springs are each of length 3l, and then released. Find the velocity of the particle when it crosses z = 0.arrow_forwardIn the figure below, a semicircular conductor of radius R = 0.260 m is rotated about the axis AC at a constant rate of 130 rev/min. A uniform magnetic field of magnitude 1.22 T fills the entire region below the axis and is directed out of the page. R Pout (a) Calculate the maximum value of the emf induced between the ends of the conductor. 1.77 v (b) What is the value of the average induced emf for each complete rotation? 0 v (c) How would your answers to parts (a) and (b) change if the magnetic field were allowed to extend a distance R above the axis of rotation? (Select all that apply.) The value in part (a) would increase. The value in part (a) would remain the same. The value in part (a) would decrease. The value in part (b) would increase. The value in part (b) would remain the same. The value in part (b) would decrease. × (d) Sketch the emf versus time when the field is as drawn in the figure. Choose File No file chosen This answer has not been graded yet. (e) Sketch the emf…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning
Stars and Galaxies (MindTap Course List)PhysicsISBN:9781337399944Author:Michael A. SeedsPublisher:Cengage Learning

 Stars and GalaxiesPhysicsISBN:9781305120785Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Stars and GalaxiesPhysicsISBN:9781305120785Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:9781337399920
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning

Stars and Galaxies (MindTap Course List)
Physics
ISBN:9781337399944
Author:Michael A. Seeds
Publisher:Cengage Learning



Stars and Galaxies
Physics
ISBN:9781305120785
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill
Time Dilation - Einstein's Theory Of Relativity Explained!; Author: Science ABC;https://www.youtube.com/watch?v=yuD34tEpRFw;License: Standard YouTube License, CC-BY