
Concept explainers
Interpretation:
The relationship between the molecules in each pair is to be determined. Whether drawings represent constitutional isomers, stereoisomers, or just different ways of drawing the same compound is to be determined. If they are stereoisomers, it is to be determined if they are enantiomers or diastereomers.
Concept introduction:
Constitutional isomers are compounds with the same chemical formula but different connectivity.
Stereoisomers are compounds with the same chemical formula and connectivity but different spatial arrangement.
If a pair of stereoisomers forms an object and non-superimposable mirror image, then they are called enantiomers. Enantiomers contain a chiral center.
If a pair of stereoisomers does not have an object and its mirror image relation, then they are called diastereomers.
Identical molecules have the same absolute configuration at each chiral center.
Answer to Problem 38P
Solution:
a) These two structures are enantiomers.
b) These are different ways of drawing the same compound.
c) These structures are enantiomers.
d) These structures are constitutional isomers.
e) These are different ways of drawing the same compound.
f) These are different ways of drawing the same compound.
g) These structures are enantiomers.
h) These are diastereomers.
i) These structures are different ways of drawing the same compound.
j) These structures are different ways of drawing the same compound.
Explanation of Solution
a) The two given structures are as follows:
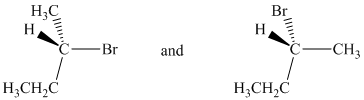
Both structures are chiral as four different atoms/groups attached to the central carbon. The absolute configurations at the chiral center for the two structures are determined from Cahn-Ingold-Prelog (CIP) system.
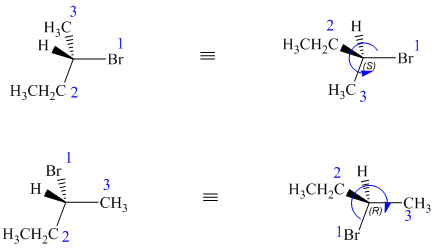
In both structures, the lowest priority group (H) is at the front. The structures are first rotated so that the hydrogen is at the back. The order of priority in the molecule is
In the first structure, the three highest ranking groups trace a counterclockwise path, giving an absolute configuration S.
In the second structure, the three highest ranking groups trace a clockwise path, giving an absolute configuration R.
Since the two structures have different absolute configurations, they are enantiomers.
b) The structures have a plane of symmetry, passing through the atoms (marked in blue) on the horizontal bonds. Rotating any one structure through

c) The two given structures are as follows:
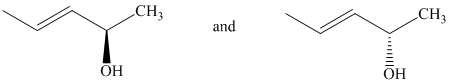
Both the given structures contain one chiral center with identical substituents.
Based on CIP system, the order of priority of the groups attached to the chiral center is

In the first structure, the hydrogen is at the back. The three highest ranking groups trace a clockwise path, giving an absolute configuration of R.
The second structure first needs to be rotated around the horizontal axis so that the hydrogen is at the back. With this, the other three groups follow a counterclockwise path, giving an absolute configuration of S.

Since the two structures have different absolute configurations at the identical chirality centers, these are enantiomers of each other.
d) The two given structures are as follows:
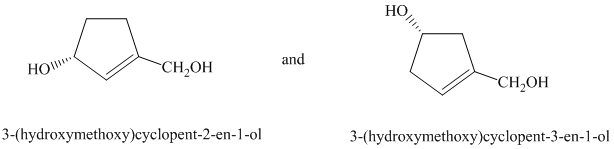
In the first structure, the carbon atom to which the hydroxyl group is attached is numbered first
In the second structure, the carbon atom to which the hydroxyl group is attached is numbered first
In both the given structures, the position of the double bond is different. Thus, the given drawings represent two different compounds with the same molecular formula but with different connectivity of atoms. Thus, they are constitutional isomers of each other.
e) These structures are identical and show different ways of drawing the same compound.

The structures have a plane of symmetry, passing through
f) The given drawings are as follows:
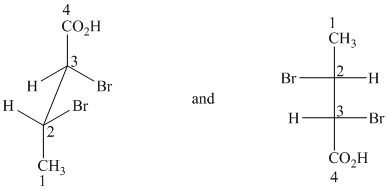
These are different ways of drawing the same compound as shown below:
Both structures contain two chiral centers, at
Before comparing, the two structures are redrawn using wedge-dash bonds.
Following the CIP system shows that the two chiral centers in both structures have the same absolute configurations (2R, 3R). Therefore, they are identical and indicate different ways of drawing the same compound.
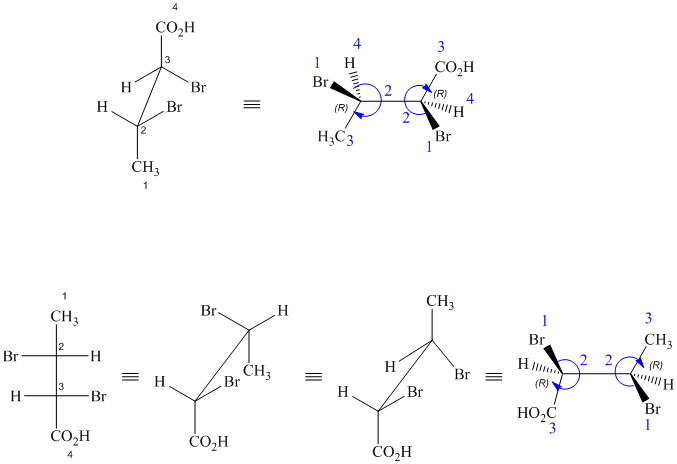
g) These structures are enantiomers.
The two drawings contain two chirality centers each. Absolute configurations at each chirality centers for these are shown below:
Both structures have two chiral centers,
Based on the CIP system, the absolute configurations of the two centers in the first structure are (2R, 3R).
The absolute configurations of the two centers in second structure are (2S, 3S).
As the absolute configurations at both the centers are changed, the structures are mirror images and therefore enantiomers.
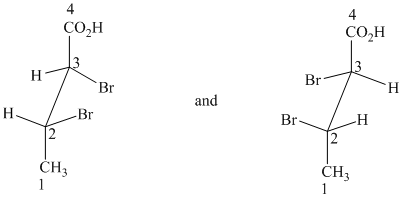
h) These structures are diastereomers – cis-trans isomers.
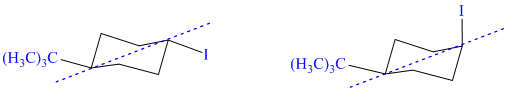
Both structures have two chiral centers. However, they also have a plane of symmetry, one that contains the isopropyl group, iodine atom, and the ring carbons to which these are bonded. Because of this plane of symmetry, these are not chiral molecules; they are diastereomers.
Additionally the iodine atom has different orientations in the two structures with respect to the isopropyl substituent. In the first structure, it is in the equatorial position, and in the second structure it is in the axial position. The isopropyl group is in the equatorial position in both. As a result, the isopropyl group and iodine are trans to each other in the first structure. They are cis to each other in the second structure.
Therefore, these two structures are diastereomers and show cis-trans isomerism.
i) The given pair of compounds are as follows:

In the first structure, the double bonded carbon atoms are
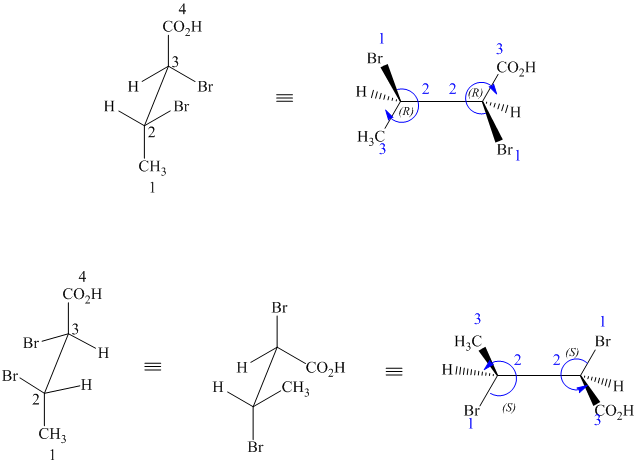
j) These structures are different ways of drawing the same compound.
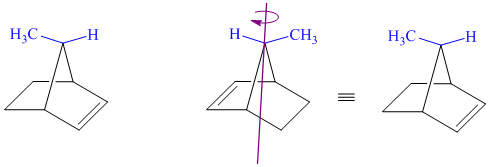
Rotating one of the structures through
Want to see more full solutions like this?
Chapter 4 Solutions
ORGANIC CHEMISTRY (LL)-W/SOLN.>CUSTOM<
- Provide the semi-developed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forwardGiven a 1,3-dicarbonyl compound (R1-CO-CH2-CO-R2), indicate the formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardAn orange laser has a wavelength of 610 nm. What is the energy of this light?arrow_forward
- The molar absorptivity of a protein in water at 280 nm can be estimated within ~5-10% from its content of the amino acids tyrosine and tryptophan and from the number of disulfide linkages (R-S-S-R) between cysteine residues: Ε280 nm (M-1 cm-1) ≈ 5500 nTrp + 1490 nTyr + 125 nS-S where nTrp is the number of tryptophans, nTyr is the number of tyrosines, and nS-S is the number of disulfide linkages. The protein human serum transferrin has 678 amino acids including 8 tryptophans, 26 tyrosines, and 19 disulfide linkages. The molecular mass of the most dominant for is 79550. Predict the molar absorptivity of transferrin. Predict the absorbance of a solution that’s 1.000 g/L transferrin in a 1.000-cm-pathlength cuvet. Estimate the g/L of a transferrin solution with an absorbance of 1.50 at 280 nm.arrow_forwardIn GC, what order will the following molecules elute from the column? CH3OCH3, CH3CH2OH, C3H8, C4H10arrow_forwardBeer’s Law is A = εbc, where A is absorbance, ε is the molar absorptivity (which is specific to the compound and wavelength in the measurement), and c is concentration. The absorbance of a 2.31 × 10-5 M solution of a compound is 0.822 at a wavelength of 266 nm in a 1.00-cm cell. Calculate the molar absorptivity at 266 nm.arrow_forward
- How to calculate % of unknown solution using line of best fit y=0.1227x + 0.0292 (y=2.244)arrow_forwardGiven a 1,3-dicarbonyl compound, state the (condensed) formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardComplete the following acid-base reactions and predict the direction of equilibrium for each. Justify your prediction by citing pK values for the acid and conjugate acid in each equilibrium. (a) (b) NHs (c) O₂N NH NH OH H₁PO₁arrow_forward
- 23.34 Show how to convert each starting material into isobutylamine in good yield. ཅ ནད ཀྱི (b) Br OEt (c) (d) (e) (f) Harrow_forwardPlease help me Please use https://app.molview.com/ to draw this. I tried, but I couldn't figure out how to do it.arrow_forwardPropose a synthesis of 1-butanamine from the following: (a) a chloroalkane of three carbons (b) a chloroalkane of four carbonsarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

