
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
9th Edition
ISBN: 9781305701021
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3.SE, Problem 38AP
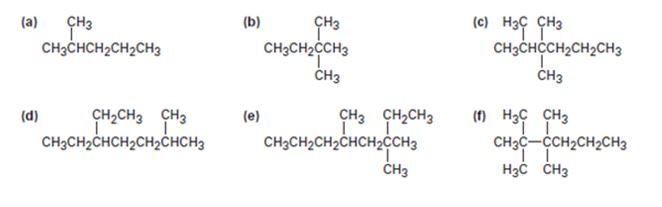
Give IUPAC names for the following compounds:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
43) 10.00 ml of vinegar (active ingredient is acetic acid) is titrated to the endpoint
using 19.32 ml of 0.250 M sodium hydroxide. What is the molarity of acetic acid
in the vinegar? YOU MUST SHOW YOUR WORK.
NOTE: MA x VA = MB x VB
424 Repon Sheet Rates of Chemical Reactions : Rate and Order of 1,0, Deception
B. Effect of Temperature
BATH TEMPERATURE
35'c
Yol of Oh
نام
Time
485
Buret rend
ing(n)
12
194
16.
6
18
20
10
22
24
14
115 95
14738
2158235
8:26 CMS
40148
Total volume of 0, collected
Barometric pressure 770-572
ml
mm Hg
Vapor pressure of water at bath temperature (see Appendix L) 42.2
Slope
Compared with the rate found for solution 1, there is
Using the ideal gas law, calculate the moles of O; collected
(show calculations)
times faster
10
Based on the moles of O, evolved, calculate the molar concentration of the original 3% 1,0, solution (sho
calculations)
Steps and explanation please
Chapter 3 Solutions
Bundle: Organic Chemistry, 9th, Loose-Leaf + OWLv2, 4 terms (24 months) Printed Access Card
Ch. 3.1 - Prob. 1PCh. 3.1 - Prob. 2PCh. 3.1 - Identify the functional groups in the following...Ch. 3.2 - Draw structures of the five isomers of C6H14.Ch. 3.2 - Propose structures that meet the following...Ch. 3.2 - Prob. 6PCh. 3.3 - Draw the eight 5-carbon alkyl groups (pentyl...Ch. 3.3 - Identify the carbon atoms in the following...Ch. 3.3 - Prob. 9PCh. 3.3 - Prob. 10P
Ch. 3.4 - Give IUPAC names for the following compounds:Ch. 3.4 - Prob. 12PCh. 3.4 - Name the eight 5-carbon alkyl groups you drew in...Ch. 3.4 - Give the IUPAC name for the following hydrocarbon,...Ch. 3.7 - Make a graph of potential energy versus angle of...Ch. 3.7 - Sight along the C2-C1 bond of 2-methylpropane...Ch. 3.7 - Sight along the C2-C3 bond of 2,3-dimethylbutane,...Ch. 3.7 - Draw a Newman projection along the C2-C3 bond of...Ch. 3.SE - Prob. 19VCCh. 3.SE - Prob. 20VCCh. 3.SE - Draw a Newman projection along the C2-C3 bond of...Ch. 3.SE - Prob. 22APCh. 3.SE - Prob. 23APCh. 3.SE - Propose structures for the following: (a) A...Ch. 3.SE - Prob. 25APCh. 3.SE - Draw the structures of the following molecules:...Ch. 3.SE - Draw structures that meet the following...Ch. 3.SE - Prob. 28APCh. 3.SE - In each of the following sets, which structures...Ch. 3.SE - There are seven constitutional isomers with the...Ch. 3.SE - Prob. 31APCh. 3.SE - Draw compounds that contain the following: (a) A...Ch. 3.SE - Prob. 33APCh. 3.SE - Draw and name all monochloro derivatives of...Ch. 3.SE - Draw structures for the following: (a)...Ch. 3.SE - Prob. 36APCh. 3.SE - Draw a compound that: (a) Has nine primary...Ch. 3.SE - Give IUPAC names for the following compounds:Ch. 3.SE - Name the five isomers of C6H14.Ch. 3.SE - Explain why each of the following names is...Ch. 3.SE - Prob. 41APCh. 3.SE - Consider 2-methylbutane (isopentane). Sighting...Ch. 3.SE - What are the relative energies of the three...Ch. 3.SE - Construct a qualitative potential-energy diagram...Ch. 3.SE - Prob. 45APCh. 3.SE - Draw the most stable conformation of pentane,...Ch. 3.SE - Draw the most stable conformation of...Ch. 3.SE - Prob. 48APCh. 3.SE - Prob. 49APCh. 3.SE - Formaldehyde, H2C=O, is known to all biologists...Ch. 3.SE - Prob. 51APCh. 3.SE - Increased substitution around a bond leads to...Ch. 3.SE - Prob. 53APCh. 3.SE - In the next chapter we'll look at...Ch. 3.SE - We’ll see in the next chapter that there are two...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use diagram to answer the following: 1.Is the overall rxn endo- or exothermic. Explain briefly your answer____________________2. How many steps in this mechanism?_____________3. Which is the rate determining step? Explain briefly your answer____________________4. Identify (circle and label) the reactants,the products and intermediate (Is a Cation, Anion, or a Radical?) Please explain and provide full understanding.arrow_forwardDraw the entire mechanism and add Curved Arrows to show clearly how electrons areredistributed in the process. Please explain and provide steps clearly.arrow_forward15) Create Lewis structure Br Brarrow_forward
- LIOT S How would you make 200. mL of a 0.5 M solution of CuSO4 5H2O from solid copper (II) sulfate? View Rubricarrow_forwardSteps and explantions pleasearrow_forwardMatch the denticity to the ligand. Water monodentate ✓ C₂O2 bidentate H₂NCH₂NHCH2NH2 bidentate x EDTA hexadentate Question 12 Partially correct Mark 2 out of 2 Flag question Provide the required information for the coordination compound shown below: Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2✔ Geometry: linear Oxidation state of transition metal ion: +3 x in 12 correct out of 2 question Provide the required information for the coordination compound shown below. Na NC-Ag-CN] Number of ligands: 20 Coordination number: 2 Geometry: linear 0 Oxidation state of transition metal ion: +3Xarrow_forward
- Can you explain step by step behind what the synthetic strategy would be?arrow_forwardPlease explain step by step in detail the reasoning behind this problem/approach/and answer. thank you!arrow_forward2. Predict the product(s) that forms and explain why it forms. Assume that any necessary catalytic acid is present. .OH HO H₂N OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY