
(a)
Interpretation:
The product, balanced equation, net ionic equation and the type of the given reaction should be identified.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
(a)
Answer to Problem 3.10CYU
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is gas forming reaction and the balanced equation is shown below
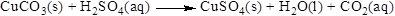
Net ionic equation of the given reaction shown below (a)
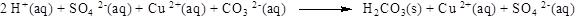
Explanation of Solution
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is gas forming reaction and the balanced equation is shown below
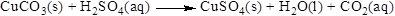
The given compound is copper carbonate and sulfuric acid which is soluble in water. In this reaction copper carbonate reaction with sulfuric acid to give copper sulfate and carbon dioxide and water.
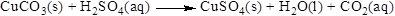
Balance the equation,
The reaction is already balanced. Therefore the balanced equation is given below.
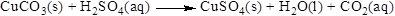
The net ionic equation is given below,
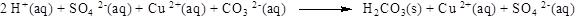
(b)
Interpretation:
The product, balanced equation, net ionic equation and the type of the given reaction should be identified.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
(b)
Answer to Problem 3.10CYU
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is oxidation-reduction reaction and the balanced equation is shown below (b)
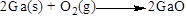
Net ionic equation of the given reaction shown below (b)
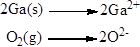
Explanation of Solution
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is oxidation-reduction reaction and the balanced equation is shown below
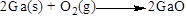
The given compound is gallium and oxygen. In this reaction gallium reaction with oxygen to give gallium oxide, sulfur dioxide. Here the oxidation state of gallium is zero in the reactant and
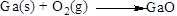
Balance the equation,
Balance the oxygen atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients. There are two oxygen atoms in the left side and one oxygen atoms in the right side. Therefore two molecule of gallium oxide is added to right side of reaction. Therefore the balanced equation is given below.
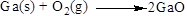
Balance the gallium atom in the given equation. There are two gallium atoms in the right side and one gallium atoms in the left side. Therefore two molecule of gallium is added to left side of reaction. Therefore the balanced equation is given below.
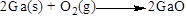
Net ionic equation of the given reaction shown below

(c)
Interpretation:
The product, balanced equation, net ionic equation and the type of the given reaction should be identified.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
(c)
Answer to Problem 3.10CYU
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is acid-base reaction and the balanced equation is shown below (c)
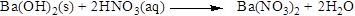
Net ionic equation of the given reaction shown below (c)
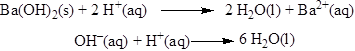
Explanation of Solution
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is acid-base reaction and the balanced equation is shown below
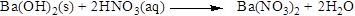
The given compound is barium hydroxide and nitric acid. In this reaction barium hydroxide reaction with nitric acid to give barium nitrate and formation of water is also the product.
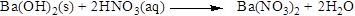
Balance the equation,
Balance the nitrogen atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients. There are two nitrogen atoms in the right side and one nitrogen atoms in the left side. Therefore two molecule of nitric acid is added to left side of reaction. Therefore the balanced equation is given below.
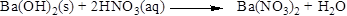
Balance the hydrogen atom in the given equation. There are two hydrogen atoms in the right side and four hydrogen atoms in the left side. Therefore two molecule of water is added to right side of reaction. Therefore the balanced equation is given below.
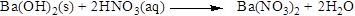
Net ionic equation of the given reaction shown below
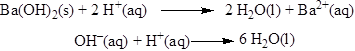
(d)
Interpretation:
The product, balanced equation, net ionic equation and the type of the given reaction should be identified.
Concept introduction:
Precipitation reaction: The formation of the product is insoluble when the ions combine in the solution is called precipitation reaction.
Acid - base reaction: Formation of the salt from the cation from the base and anion from the acid and formation of water is also the product.
Gas forming reaction: The reaction of acid and metal carbonates which produce carbonic acid. The carbonic acid decomposes which gives water and carbon dioxide.
Oxidation - reduction reaction: The electrons are transferred to one to other is called oxidation reduction reaction.
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides,
(d)
Answer to Problem 3.10CYU
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is precipitation reaction and the balanced equation is shown below.

Net ionic equation of the given reaction shown below (a)
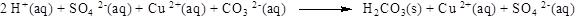
Explanation of Solution
The product of the reaction, balancing of the reaction and the type of the reaction is shown below, the given reaction is precipitation reaction and the balanced equation is shown below

The given compound is copper chloride and ammonium sulfide. In this reaction copper chloride reaction with ammonium sulfide to give copper sulfide and ammonium chloride.
the given reaction is precipitation reaction.

Balance the equation,
Balance the nitrogen atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients. There are two nitrogen atoms in the left side and one nitrogen atoms in the right side. Therefore two molecule of ammoniu chloride is added to right side of reaction. Therefore the balanced equation is given below.

Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry & Chemical Reactivity
- Identify and provide an explanation that distinguishes a qualitative and quantitative chemical analysis. Provide examples.arrow_forwardIdentify and provide an explanation of the operational principles behind a Atomic Absorption Spectrometer (AAS). List the steps involved.arrow_forwardInstructions: Complete the questions in the space provided. Show all your work 1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure the initial reaction rate and the starting concentrations of the reactions for 4 trials. BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l) Initial rate Trial [BrO3] [H*] [Br] (mol/L) (mol/L) | (mol/L) (mol/L.s) 1 0.10 0.10 0.10 8.0 2 0.20 0.10 0.10 16 3 0.10 0.20 0.10 16 4 0.10 0.10 0.20 32 a. Based on the above data what is the rate law expression? b. Solve for the value of k (make sure to include proper units) 2. The proposed reaction mechanism is as follows: i. ii. BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq) HBrO³ (aq) + H* (aq) → H₂BrO3* (aq) iii. H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l) [Fast] [Medium] [Slow] iv. Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l) [Fast] Evaluate the validity of this proposed reaction. Justify your answer.arrow_forward
- a. H3C CH3 H, 1.0 equiv. Br2arrow_forwardH3C. H3C CH 3 CH 3 CH3 1. LDA 2. PhSeCl 3. H2O2arrow_forwardPlease predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forward
- in the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forwardIf we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





