
Concept explainers
(a)
Interpretation:
The dissociation constant of protein and antibody A is to be calculated.
Concept introduction:
The protein is made up of different amino acids. The amino acid is the smallest unit of a protein. The protein may be composed of primary, secondary, tertiary and quarternary structure. The antibody is also a type of protein which is derived by plasma cells and it is used by the immune system of the cells.
Answer to Problem 19P
The dissociation constant of a complex between protein and antibody A is
Explanation of Solution
The dissociation constant is defined as the breaking of larger molecules into its smaller units or it is defined as the ratio of products to the reactants with respect to their stoichiometric number.
The reaction corresponding to the dissociation of PA is given below.
The dissociation constant of the above equation is shown below.
The value of dissociation constant is obtained when half of the complex is dissociated as given below.
This is the concentration of protein which is obtained and the concentration of the complex which is dissociated.
The concentration of antibody A is obtained from the graph shown below.
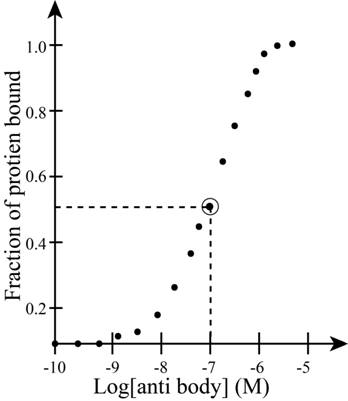
Figure 1
From the graph above.
Substitute values of
Therefore the dissociation constant the complex between protein and antibody A is
(b)
Interpretation:
The dissociation constant of protein and antibody B is to be calculated.
Concept introduction:
The protein is made up of different amino acids. The amino acid is the smallest unit of a protein. The protein may be composed of primary, secondary, tertiary and quarternary structure. The antibody is also a type of protein which is derived by plasma cells and it is used by the immune system of the cells.
Answer to Problem 19P
The dissociation constant of a complex between protein and antibody B is
Explanation of Solution
The dissociation constant is defined as the breaking of larger molecules into its smaller units or it is defined as the ratio of products to the reactants with respect to their stoichiometric number.
Consider a reaction given below.
The dissociation constant of the above equation is shown below.
The value of dissociation constant is obtained when half of the complex is dissociated as given below.
This is the concentration of protein which is obtained and the concentration of the complex which is dissociated.
The concentration of antibody B is obtained from the graph shown below.
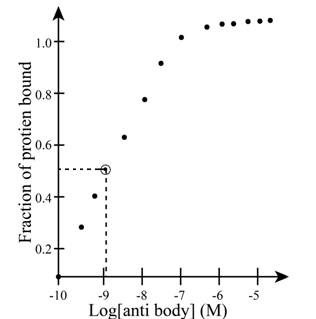
Figure 2
From the graph above, the concentration of the antibody is calculated as follows:
Substitute values of
Therefore the dissociation constant the complex between protein and antibody B is
On comparing, it is found that antibody A and B are identical except for one single amino acid. The reason for the generation of antibody B is through gene mutation which permanentlychanges the DNA sequence of a species. It may be hereditary or acquired mutation. Since in this case only one amino acid changes.
Therefore, the mutation is a point mutationthat generated antibody B.
Want to see more full solutions like this?
Chapter 35 Solutions
SAPLINGPLUS F/BIOCHEM+ICLICKER REEF-CODE
- please draw it for me and tell me where i need to modify the structurearrow_forwardPlease help determine the standard curve for my Kinase Activity in Excel Spreadsheet. Link: https://mnscu-my.sharepoint.com/personal/vi2163ss_go_minnstate_edu/_layouts/15/Doc.aspx?sourcedoc=%7B958f5aee-aabd-45d7-9f7e-380002892ee0%7D&action=default&slrid=9b178ea1-b025-8000-6e3f-1cbfb0aaef90&originalPath=aHR0cHM6Ly9tbnNjdS1teS5zaGFyZXBvaW50LmNvbS86eDovZy9wZXJzb25hbC92aTIxNjNzc19nb19taW5uc3RhdGVfZWR1L0VlNWFqNVc5cXRkRm4zNDRBQUtKTHVBQldtcEtWSUdNVmtJMkoxQzl3dmtPVlE_cnRpbWU9eEE2X291ZHIzVWc&CID=e2126631-9922-4cc5-b5d3-54c7007a756f&_SRM=0:G:93 Determine the amount of VRK1 is present 1. Average the data and calculate the mean absorbance for each concentration/dilution (Please over look for Corrections) 2. Blank Correction à Subtract 0 ug/mL blank absorbance from all readings (Please over look for Corrections) 3. Plot the Standard Curve (Please over look for Corrections) 4. Convert VRK1 concentration from ug/mL to g/L 5. Use the molar mass of VRK1 to convert to M and uM…arrow_forwardMacmillan Learning Cholesterol synthesis begins with the formation of mevalonate from acetyl CoA. This process activates mevalonate and converts it to isopentenyl pyrophosphate. Identify the atoms in mevalonate and isopentenyl pyrophosphate that will be labeled from acetyl CoA labeled with 14C in the carbonyl carbon. Place 14C atoms and C atoms to denote which carbon atoms are labeled and which are not labeled. H₂C COA 14C-labeled acetyl-CoA HHH [c] H H OH 014C - OH H HH H Mevalonate CH3 H H 14C H Η H H Incorrect Answer of o -P-O-P-0- Isopentenyl pyrophosphate с Answer Bank 14Carrow_forward
- Draw the reaction between sphingosine and arachidonic acid. Draw out the full structures.arrow_forwardDraw both cis and trans oleic acid. Explain why cis-oleic acid has a melting point of 13.4°C and trans-oleic acid has a melting point of 44.5°C.arrow_forwardDraw the full structure of the mixed triacylglycerol formed by the reaction of glycerol and the fatty acids arachidic, lauric and trans-palmitoleic. Draw the line structure.arrow_forward
- Draw out the structure for lycopene and label each isoprene unit. "Where is lycopene found in nature and what health benefits does it provide?arrow_forwardWhat does it mean to be an essential fatty acid? What are the essential fatty acids?arrow_forwardCompare and contrast primary and secondary active transport mechanisms in terms of energy utilisation and efficiency. Provide examples of each and discuss their physiological significance in maintaining ionic balance and nutrient uptake. Rubric Understanding the key concepts (clearly and accurately explains primary and secondary active transport mechanisms, showing a deep understanding of their roles) Energy utilisation analysis ( thoroughly compares energy utilisation in primary and secondary transport with specific and relevant examples Efficiency discussion Use of examples (provides relevant and accurate examples (e.g sodium potassium pump, SGLT1) with clear links to physiological significance. Clarity and structure (presents ideas logically and cohesively with clear organisation and smooth transition between sections)arrow_forward
- 9. Which one of the compounds below is the major organic product obtained from the following reaction sequence, starting with ethyl acetoacetate? 요요. 1. NaOCH2CH3 CH3CH2OH 1. NaOH, H₂O 2. H3O+ 3. A OCH2CH3 2. ethyl acetoacetate ii A 3. H3O+ OH B C D Earrow_forward7. Only one of the following ketones cannot be made via an acetoacetic ester synthesis. Which one is it? Ph کہ A B C D Earrow_forward2. Which one is the major organic product obtained from the following reaction sequence? HO A OH 1. NaOEt, EtOH 1. LiAlH4 EtO OEt 2. H3O+ 2. H3O+ OH B OH OH C -OH HO -OH OH D E .CO₂Etarrow_forward
 Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning
Human Heredity: Principles and Issues (MindTap Co...BiologyISBN:9781305251052Author:Michael CummingsPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning





