
(a)
The energy of the ground state
(a)
Answer to Problem 41P
The energy of the ground state
Explanation of Solution
Given:
The length of one-dimensional box is
Formula used:
The expression for energy of ground state is given by,
The expression for energy of nth states is given by,
Calculation:
The energy of ground stateis calculated as,
Solve further as,
The energy of first excited state is calculated as,
The energy of second excited state is calculated as,
Solve further as,
The energy level diagram of the system is shown in Figure 1.
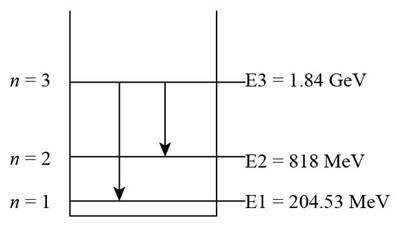
Figure 1
Conclusion:
Therefore, the energy of the ground state
(b)
The wavelength of
(b)
Answer to Problem 41P
The wavelength of electromagnetic radiation emittedis
Explanation of Solution
Given:
The neutron makes transition from
Formula used:
The expression for wavelength of electromagnetic radiation emitted is given by,
Calculation:
The wavelength of electromagnetic radiation emitted is calculated as,
Solve further as,
Conclusion:
Therefore, the wavelength of electromagnetic radiation emitted is
(c)
The wavelength of electromagnetic radiation emitted.
(c)
Answer to Problem 41P
The wavelength of electromagnetic radiation emitted is
Explanation of Solution
Given:
The neutron makes transition from
Formula used:
The expression for wavelength of electromagnetic radiation emitted is given by,
Calculation:
The wavelength of electromagnetic radiation emitted is calculated as,
Solve further as,
Conclusion:
Therefore, the wavelength of electromagnetic radiation emitted is
(d)
The wavelength of electromagnetic radiation emitted.
(d)
Answer to Problem 41P
The wavelength of electromagnetic radiation emitted is
Explanation of Solution
Given:
The neutron makes transition from
Formula used:
The expression for wavelength of electromagnetic radiation emitted is given by,
Calculation:
The wavelength of electromagnetic radiation emitted is calculated as,
Solve further as,
Conclusion:
Therefore, the wavelength of electromagnetic radiation emitted is
Want to see more full solutions like this?
Chapter 34 Solutions
Physics for Scientists and Engineers
- Required information Two speakers vibrate in phase with each other at 523 Hz. At certain points in the room, the sound waves from the two speakers interfere destructively. One such point is 1.45 m from speaker #1 and is between 2.00 m and 4.00 m from speaker #2. The speed of sound in air is 343 m/s. How far is this point from speaker #2? marrow_forwarda) Consider the following function, where A is a constant. y(x,t) = A(x — vt). Can this represent a wave that travels along? Explain. b) Which of the following are possible traveling waves, provide your reasoning and give the velocity of the wave if it can be a traveling wave. e-(a²x²+b²²-2abtx b.1) y(x,t) b.2) y(x,t) = = A sin(ax² - bt²). 2 b.3) y(x,t) = A sin 2π (+) b.4) y(x,t) = A cos² 2π(t-x). b.5) y(x,t) = A cos wt sin(kx - wt)arrow_forwardThe capacitor in (Figure 1) is initially uncharged. The switch is closed at t=0. Immediately after the switch is closed, what is the current through the resistor R1, R2, and R3? What is the final charge on the capacitor? Please explain all steps.arrow_forward
- Suppose you have a lens system that is to be used primarily for 620-nm light. What is the second thinnest coating of fluorite (calcium fluoride) that would be non-reflective for this wavelength? × nm 434arrow_forwardThe angle between the axes of two polarizing filters is 19.0°. By how much does the second filter reduce the intensity of the light coming through the first? I = 0.106 40 xarrow_forwardAn oil slick on water is 82.3 nm thick and illuminated by white light incident perpendicular to its surface. What color does the oil appear (what is the most constructively reflected wavelength, in nanometers), given its index of refraction is 1.43? (Assume the index of refraction of water is 1.33.) wavelength color 675 × nm red (1 660 nm)arrow_forward
- A 1.50 μF capacitor is charging through a 16.0 Ω resistor using a 15.0 V battery. What will be the current when the capacitor has acquired 1/4 of its maximum charge? Please explain all stepsarrow_forwardIn the circuit shown in the figure (Figure 1), the 6.0 Ω resistor is consuming energy at a rate of 24 J/s when the current through it flows as shown. What are the polarity and emf of the battery E, assuming it has negligible internal resistance? Please explain all steps. I know you need to use the loop rule, but I keep getting the answer wrong.arrow_forwardIf you connect a 1.8 F and a 2.6 F capacitor in series, what will be the equivalent capacitance?arrow_forward
- Suppose that a particular heart defibrillator uses a 1.5 x 10-5 Farad capacitor. If it is charged up to a voltage of 7300 volts, how much energy is stored in the capacitor? Give your answer as the number of Joules.arrow_forwardThe voltage difference across an 8.3 nanometer thick cell membrane is 6.5 x 10-5volts. What is the magnitude of the electric field inside this cell membrane? (Assume the field is uniform, and give your answer as the number of Volts per meter... which is the same as the number of Newtons per Coulomb.)arrow_forwardThree identical capacitors are connected in parallel. When this parallel assembly of capacitors is connected to a 12 volt battery, a total of 3.1 x 10-5 coulombs flows through the battery. What is the capacitance of one individual capacitor? (Give your answer as the number of Farads.)arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax
University Physics Volume 3PhysicsISBN:9781938168185Author:William Moebs, Jeff SannyPublisher:OpenStax Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning
Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning





