
EBK ORGANIC CHEMISTRY
9th Edition
ISBN: 9780100591318
Author: McMurry
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30.SE, Problem 14MP
Plastic photochromic sunglasses are based on the following reversible rearrangement of a dye inside the lenses that occurs when the lenses are exposed to sunlight. The original dye absorbs UV light but not visible light and is thus colorless, while the rearrangement product absorbs visible light and is thus darkened.
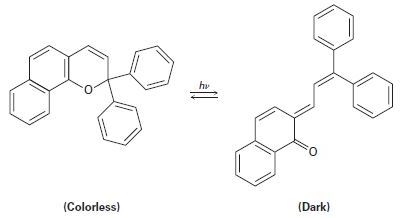
(a) Show the mechanism of the rearrangement.
(b) Why does the rearrangement product absorb at a longer wavelength (visible light) than the original dye (UV)?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a reasonable mechanism for the following reaction:
Draw the mechanism for the following reaction:
CH3
CH3
Et-OH
Et
Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the
electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created.
H± EXP.
L
CONT.
י
Α
[1]
осн
CH3
а
CH3
:Ö
Et
H
0
N
о
S
0
Br
Et-ÖH
|
P
LL
F
20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?
Chapter 30 Solutions
EBK ORGANIC CHEMISTRY
Ch. 30.1 - Prob. 1PCh. 30.3 - Prob. 2PCh. 30.3 - Prob. 3PCh. 30.4 - Prob. 4PCh. 30.6 - What stereochemistry would you expect for the...Ch. 30.6 - Prob. 6PCh. 30.7 - Prob. 7PCh. 30.8 - Propose a mechanism to account for the fact that...Ch. 30.8 - When a 2, 6-disubstituted allyl phenyl ether is...Ch. 30.9 - Prob. 10P
Ch. 30.SE - Predict the product obtained when the following...Ch. 30.SE - Prob. 12VCCh. 30.SE - The following rearrangement of N-allyl-N,...Ch. 30.SE - Plastic photochromic sunglasses are based on the...Ch. 30.SE - Prob. 15MPCh. 30.SE - Prob. 16MPCh. 30.SE - Prob. 17MPCh. 30.SE - Prob. 18APCh. 30.SE - Prob. 19APCh. 30.SE - Prob. 20APCh. 30.SE - Prob. 21APCh. 30.SE - Prob. 22APCh. 30.SE - Prob. 23APCh. 30.SE - Prob. 24APCh. 30.SE - Prob. 25APCh. 30.SE - Prob. 26APCh. 30.SE - Prob. 27APCh. 30.SE - Prob. 28APCh. 30.SE - Propose a pericyclic mechanism to account for the...Ch. 30.SE - Prob. 30APCh. 30.SE - Prob. 31APCh. 30.SE - Prob. 32APCh. 30.SE - Prob. 33APCh. 30.SE - Bicyclohexadiene, also known as Dewar benzene, is...Ch. 30.SE - Prob. 35APCh. 30.SE - Prob. 36APCh. 30.SE - The 1H NMR spectrum of bullvalene at 100 C...Ch. 30.SE - Prob. 38APCh. 30.SE - Prob. 39APCh. 30.SE - Prob. 40APCh. 30.SE - In light of your answer to Problem 30-40, explain...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the pH of 0.015 M HCl.arrow_forwardCalculate the pH of 0.450 M KOH.arrow_forwardWhich does NOT describe a mole? A. a unit used to count particles directly, B. Avogadro’s number of molecules of a compound, C. the number of atoms in exactly 12 g of pure C-12, D. the SI unit for the amount of a substancearrow_forward
- 5 What would the complete ionic reaction be if aqueous solutions of potassium sulfate and barium acetate were mixed? ed of Select one: O a 2 K SO4 + Ba2 +2 C₂H3O21 K+SO4 + Ba2+ + 2 C2H3O21 K+SO42 + Ba2 +2 C2H3O2 BaSO4 +2 K+ + 2 C2H3O estion Ob. O c. Od. 2 K SO4 +Ba2 +2 C₂H₂O₂ BaSO4 + K+ + 2 C2H3O BaSO4 + K + 2 C2H301 →Ba² +SO42 +2 KC2H3O s pagearrow_forward(28 pts.) 7. Propose a synthesis for each of the following transformations. You must include the reagents and product(s) for each step to receive full credit. The number of steps is provided. (OC 4) 4 steps 4 steps OH b.arrow_forwardLTS Solid: AT=Te-Ti Trial 1 Trial 2 Trial 3 Average ΔΗ Mass water, g 24.096 23.976 23.975 Moles of solid, mol 0.01763 001767 0101781 Temp. change, °C 2.9°C 11700 2.0°C Heat of reaction, J -292.37J -170.473 -193.26J AH, kJ/mole 16.58K 9.647 kJ 10.85 kr 16.58K59.64701 KJ mol 12.35k Minimum AS, J/mol K 41.582 mol-k Remember: q = mCsAT (m = mass of water, Cs=4.184J/g°C) & qsin =-qrxn & Show your calculations for: AH in J and then in kJ/mole for Trial 1: qa (24.0969)(4.1845/g) (-2.9°C)=-292.37J qsin = qrxn = 292.35 292.37J AH in J = 292.375 0.2923kJ 0.01763m01 =1.65×107 AH in kJ/mol = = 16.58K 0.01763mol mol qrx Minimum AS in J/mol K (Hint: use the average initial temperature of the three trials, con Kelvin.) AS=AHIT (1.65×10(9.64×103) + (1.0 Jimaiarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY