
ORGANIC CHEMISTRY W/OWL
9th Edition
ISBN: 9781305717527
Author: McMurry
Publisher: CENGAGE C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30.8, Problem 9P
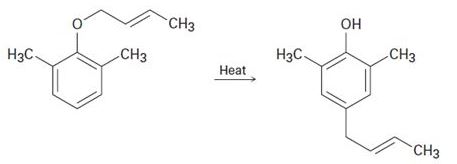
When a 2, 6-disubstituted allyl phenyl ether is heated in an attempted Claisen rearrangement, migration occurs to give the p-allyl product as the result of two sequential pericyclic reactions. Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
a. Write the product For each of the
Following reactions
H
6-836-6
레
+H₂ N
A
H
A-C-C=C-C-CH + 2 Na +2 NH3 -
H H
b. Write the reaction Mechanism For.
reaction
each
help draw the molecule
How to draw this claisen condensation reaction mechanisms/
Chapter 30 Solutions
ORGANIC CHEMISTRY W/OWL
Ch. 30.1 - Prob. 1PCh. 30.3 - Prob. 2PCh. 30.3 - Prob. 3PCh. 30.4 - Prob. 4PCh. 30.6 - What stereochemistry would you expect for the...Ch. 30.6 - Prob. 6PCh. 30.7 - Prob. 7PCh. 30.8 - Propose a mechanism to account for the fact that...Ch. 30.8 - When a 2, 6-disubstituted allyl phenyl ether is...Ch. 30.9 - Prob. 10P
Ch. 30.SE - Predict the product obtained when the following...Ch. 30.SE - Prob. 12VCCh. 30.SE - The following rearrangement of N-allyl-N,...Ch. 30.SE - Plastic photochromic sunglasses are based on the...Ch. 30.SE - Prob. 15MPCh. 30.SE - Prob. 16MPCh. 30.SE - Prob. 17MPCh. 30.SE - Prob. 18APCh. 30.SE - Prob. 19APCh. 30.SE - Prob. 20APCh. 30.SE - Prob. 21APCh. 30.SE - Prob. 22APCh. 30.SE - Prob. 23APCh. 30.SE - Prob. 24APCh. 30.SE - Prob. 25APCh. 30.SE - Prob. 26APCh. 30.SE - Prob. 27APCh. 30.SE - Prob. 28APCh. 30.SE - Propose a pericyclic mechanism to account for the...Ch. 30.SE - Prob. 30APCh. 30.SE - Prob. 31APCh. 30.SE - Prob. 32APCh. 30.SE - Prob. 33APCh. 30.SE - Bicyclohexadiene, also known as Dewar benzene, is...Ch. 30.SE - Prob. 35APCh. 30.SE - Prob. 36APCh. 30.SE - The 1H NMR spectrum of bullvalene at 100 C...Ch. 30.SE - Prob. 38APCh. 30.SE - Prob. 39APCh. 30.SE - Prob. 40APCh. 30.SE - In light of your answer to Problem 30-40, explain...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forwardHow can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forward
- Please provide the mechanism for this reacitonarrow_forwardQuestion 5: Name the following compound in two ways using side chain and using prefix amine (Common name and IUPAC name both) CH3NH2 CH3CH2NHCH3 CH₂CH₂N(CH3)2 Draw the structure of diethyl methyl amine Question 6. Write the balanced combustion reaction for: a. Hexane b. Propyne c. 2-pentene Question 7: Write the following electrophilic substitution reactions of benzene: Hint: Use notes if you get confused a. Halogenation reaction: b. Nitration reaction : c. Sulphonation reaction: d. Alkylation reaction: e. Aceylation reaction:arrow_forwardQuestion 4. Name the following structures ○ CH3-C-N-H H CH3CH2-C-N-H H CH3CH2-C-N-CH3 Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY