
Interpretation:
The suprafacial or antarafacial stereochemistry of the following sigmatropic reaction by order [x, y] is,
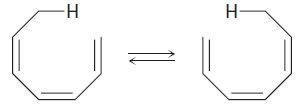
Concept introduction:
A sigmatropic rearrangement is a process in which a sigma-bonded substituent atom or group migrates across a pie electron system from one position to another. So, A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond.
Sigmatropic rearrangements are concisely described by an order term [i, j], which is defined as the migration of a σ-bond adjacent to one or more π systems to a new position (i-1) and (j-1) atoms removed from the original location of the σ-bond. [3] When the sum of i and j is an even number, this is an indication of the involvement of a neutral, all C atom chain. An odd number is an indication of the involvement of a charged C atom or of a heteroatom lone pair replacing a C = C double bond. Thus, [1, 5] and [3, 3] shifts become [1, 4] and [2, 3] shifts with heteroatoms, while preserving symmetry considerations.
If the migrating group remains on the same face of the π system, the shift is known as suprafacial, while if the migrating group transfers to the opposite face is called an antarafacial shift.
Trending nowThis is a popular solution!

Chapter 30 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- Indicate the products obtained from the reaction of 2-nitrophenol with a sulfonitric acid mixture (H2SO4 + HNO3). Indicate the majority if necessary.arrow_forwardIn organic chemistry, what is the correct name for the mixture H2SO4 + HNO3 used in reactions: sulphonitric mixture or sulfonitric mixture?arrow_forwardFormulate the products obtained by reacting p-toluidine with a sulfonate mixture. Indicate the majority if necessary.arrow_forward
- Consider this organic reaction: OH Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. x 0: の Carrow_forwardExplain the reasons for a compound's greater or lesser reactivity toward electrophilic aromatic substitution. Give reasons.arrow_forwardDraw the products of a reaction of the following alkyle chloride, shown below in the 3D ball and stick model with NaSCH3. Ignore inorganic byproducts. In the figure, a gray ball indicates a carbon atom a white ball indicates a hydrogen atom anda agreen ball indicated a chlorine atomarrow_forward
- Draw the most stable cations formed in the mass spectrometer by a deavage of the following compound Draw the most stable cations formed in the mass spectrometer by a cleavage of the following compound онarrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting anand product sytucutrs, draw the curved electron-pusing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bind-making stepsarrow_forwardDraw the major elimination and substitution products formed in this reavtion. Use a dash or wedge bond to indicatr the stereochemistry of substituents on assymetric centers, wheere applicable. Ignore any inorganic byproducts.arrow_forward
- Draw the two possible products produced in this E2 elimination. Ignore any inorganic byproductsarrow_forwardDraw the major products of this SN1 reaction. Ignore any inorganic byproducts.arrow_forwardDraw the major elimination and substitution products formed in this reaction. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, wehre applicable. Ignore and inorganic byproducts.arrow_forward
