
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
3rd Edition
ISBN: 8220101460288
Author: Deal
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 3IA.8Q
Using a dipole moment arrow (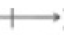 ), indicate the overall molecular polarity for the following molecules (you may have to draw out Lewis structures to do this). If the molecule is nonpolar, state no dipole.
), indicate the overall molecular polarity for the following molecules (you may have to draw out Lewis structures to do this). If the molecule is nonpolar, state no dipole.
- a. HBr
- b. OCS
- c. CH3F
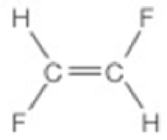
- d. CH2Br2
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What is the molarity of a 0.393 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 g/mol
The rate constant for the decay of a radioactive element is 2.28 × 10⁻³ day⁻¹. What is the half-life of this element in days?
Handwritten please
Chapter 3 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
Ch. 3 - How many electrons are in each energy level of the...Ch. 3 - How many electrons are in each energy level of the...Ch. 3 - How many valence electrons are present in the...Ch. 3 - How many valence electrons are present in the...Ch. 3 - Which of the following elements are stable as...Ch. 3 - Which of the following elements are stable as...Ch. 3 - Prob. 3.7PPCh. 3 - Prob. 3.8PPCh. 3 - Prob. 3.9PPCh. 3 - How are the names of a transition metal atom and...
Ch. 3 - Provide the charge on each element when an ion is...Ch. 3 - Provide the charge on each element when an ion is...Ch. 3 - Prob. 3.13PPCh. 3 - How many protons and electrons are present in the...Ch. 3 - Name the ions in Problem 3.13.Ch. 3 - Prob. 3.16PPCh. 3 - Prob. 3.17PPCh. 3 - Give the name and symbol of the ion with the...Ch. 3 - Prob. 3.19PPCh. 3 - Name the following ions: a. Cu2+ b. SO42 c. HPO42Ch. 3 - Prob. 3.21PPCh. 3 - Prob. 3.22PPCh. 3 - Prob. 3.23PPCh. 3 - Prob. 3.24PPCh. 3 - Prob. 3.25PPCh. 3 - Prob. 3.26PPCh. 3 - Prob. 3.27PPCh. 3 - Prob. 3.28PPCh. 3 - Draw the correct Lewis structure for each of the...Ch. 3 - Draw the correct Lewis structure for each of the...Ch. 3 - Draw the correct Lewis structure for each of the...Ch. 3 - Draw the correct Lewis structure for each of the...Ch. 3 - Determine whether each of the following is a...Ch. 3 - Determine whether each of the following is a...Ch. 3 - Prob. 3.35PPCh. 3 - Prob. 3.36PPCh. 3 - Compare (a) the number of atoms and (b) the number...Ch. 3 - Compare (a) the number of atoms and (b) the number...Ch. 3 - Calculate the following: a. the number of Na atoms...Ch. 3 - Calculate the following: a. the number of S atoms...Ch. 3 - Prob. 3.41PPCh. 3 - Determine the molar mass for the following...Ch. 3 - Prob. 3.43PPCh. 3 - Prob. 3.44PPCh. 3 - For the molecules shown, indicate whether the...Ch. 3 - For the molecules shown, indicate whether the...Ch. 3 - For the molecules in 3.45, determine the shape...Ch. 3 - For the molecules in 3.46, determine the around...Ch. 3 - Prob. 3.49PPCh. 3 - Prob. 3.50PPCh. 3 - Prob. 3.51PPCh. 3 - For each of the following molecules, (1) draw the...Ch. 3 - Prob. 3.53APCh. 3 - Prob. 3.54APCh. 3 - Prob. 3.55APCh. 3 - How many valence electrons are present in the...Ch. 3 - Prob. 3.57APCh. 3 - Prob. 3.58APCh. 3 - Complete the following statements: a. An anion has...Ch. 3 - Prob. 3.60APCh. 3 - Prob. 3.61APCh. 3 - Prob. 3.62APCh. 3 - Prob. 3.63APCh. 3 - Prob. 3.64APCh. 3 - Prob. 3.65APCh. 3 - Prob. 3.66APCh. 3 - Each of the following ions is isoelectronic with a...Ch. 3 - Each of the following ions is isoelectronic with a...Ch. 3 - Prob. 3.69APCh. 3 - Prob. 3.70APCh. 3 - Give the formula for the ionic compound formed by...Ch. 3 - Prob. 3.72APCh. 3 - Prob. 3.73APCh. 3 - Give the formula for each of the following ionic...Ch. 3 - Name the following ionic compounds: a. Na2O b....Ch. 3 - Prob. 3.76APCh. 3 - Prob. 3.77APCh. 3 - Prob. 3.78APCh. 3 - Prob. 3.79APCh. 3 - Prob. 3.80APCh. 3 - Prob. 3.81APCh. 3 - Prob. 3.82APCh. 3 - Prob. 3.83APCh. 3 - Prob. 3.84APCh. 3 - Prob. 3.85APCh. 3 - Explain the difference between a Lewis structure...Ch. 3 - Draw a Lewis structure for each of the following...Ch. 3 - Draw a Lewis structure for each of the following...Ch. 3 - Prob. 3.89APCh. 3 - Give the name of each of the following covalent...Ch. 3 - Explain the difference between an ionic bond and a...Ch. 3 - What are the units of Avogadros number?Ch. 3 - Prob. 3.93APCh. 3 - Prob. 3.94APCh. 3 - What is the mass of 4.00 moles of the following?...Ch. 3 - How many atoms or molecules are in 5.0 moles of...Ch. 3 - A pencil mark (made with graphite, a form of...Ch. 3 - Prob. 3.98APCh. 3 - Prob. 3.99APCh. 3 - Prob. 3.100APCh. 3 - Aspartic acid, a naturally occurring amino acid...Ch. 3 - Cyanoacrylic acid is one of the compounds used to...Ch. 3 - Methyl isocyanate is used in the manufacturing of...Ch. 3 - Vinyl acetate is used in the production of safety...Ch. 3 - Identify the more electronegative atom in each of...Ch. 3 - Identify the more electronegative atom in each of...Ch. 3 - Prob. 3.107APCh. 3 - Prob. 3.108APCh. 3 - Prob. 3.109CPCh. 3 - Prob. 3.110CPCh. 3 - Prob. 3.111CPCh. 3 - Vinyl chloride, C2H3Cl, is used in the production...Ch. 3 - One of the most common compounds used in...Ch. 3 - Prob. 1IA.1QCh. 3 - Prob. 1IA.2QCh. 3 - Prob. 1IA.3QCh. 3 - Prob. 1IA.4QCh. 3 - Prob. 1IA.5QCh. 3 - Prob. 1IA.6QCh. 3 - Prob. 1IA.7QCh. 3 - Prob. 1IA.8QCh. 3 - Prob. 2IA.1QCh. 3 - Complete the following table: Molecular Formula...Ch. 3 - Complete the following table: Molecular Formula...Ch. 3 - Based on the tables in questions 2 and 3, does the...Ch. 3 - Prob. 3IA.1QCh. 3 - Prob. 3IA.2QCh. 3 - Draw the Lewis structure for ammonia. NH3. Show...Ch. 3 - Draw the Lewis structure for H2O. Show the...Ch. 3 - Draw the Lewis structure for carbon dioxide. What...Ch. 3 - Draw the Lewis structure for carbon tetrachloride,...Ch. 3 - Prob. 3IA.7QCh. 3 - Using a dipole moment arrow (), indicate the...Ch. 3 - Find out which cations and anions are important in...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose the best reagents to complete the following reaction. i H A B 1. CH3CH2Na 2. H3O+ 1. CH3CH2MgBr 2. H3O+ 1. CH3MgBr Q C 2. H3O+ 1. H3O+ D 2. CH3MgBr 00 OH Q E CH³MgBrarrow_forwardThe kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. What is the half-life for this reaction?arrow_forwardChoose the best reagents to complete the following reaction. 1. PhNa A 2. H3O+ 1. PhCH2MgBr B 2. H3O+ хё 1. PhMgBr C 2. H3O+ 00 HO Q E D 1. H3O+ 2. PhMgBr PhMgBrarrow_forward
- Please answer all of the questions and provide detailed explanations and include a drawing to show the different signals on the molecule and include which ones should be highlighted.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 1 attempt remaining 1. LiAlH4 2. H3O+ Q OH ☑ Select to Drawarrow_forwardHow should I graph my data for the Absorbance of Pb and Fe for each mushroom? I want to compare the results to the known standard curve. Software: Excel Spreadsheets Link: https://mnscu-my.sharepoint.com/:x:/g/personal/vi2163ss_go_minnstate_edu/Eb2PfHdfEtBJiWh0ipHZ_kkBW4idWWwvpLPPtqoq2WkgbQ?rtime=HxrF0_tR3Ugarrow_forward
- Provide the proper IUPAC name only for the following compound. Dashes, commas, and spaces must be used correctly, but do not use italics in Canvas.arrow_forwardThe kinetics of a gas phase reaction of the form A → Products results in a rate constant of 0.00781 M/min. For this reaction, the initial concentration of A is 0.501 M. How many minutes will it take for the concentration of A to reach 0.144 Marrow_forwardWhat is the rate for the second order reaction A → Products when [A] = 0.256 M? (k = 0.761 M⁻¹s⁻¹)arrow_forward
- For reaction N2(g) + O2(g) --> 2NO(g) Write the rate of the reaction in terms of change of NO.arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forwardThe reaction of 2-oxacyclopentanone with hydrochloric acid in water (i.e., "excess") produces which of the following carboxylic acids?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY