
Concept explainers
Answer the following for water samples A and B Shown in diagrams: (3.1, 3.2, 3.3, 3.5)
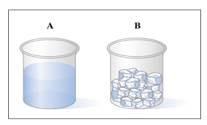
a. In which sample (A or B) does the water have its own shape?
b. Which diagram (1 or 2 or 3) represents the arrangement of particles in water sample A?
c. Which diagram (1 or 2 or 3) represents arrangement of particles in water B?
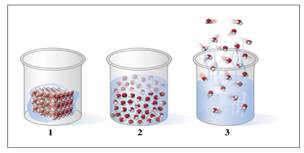
Answer the following for diagrams 1, 2, and 3:
d. The
e. The motion of the particles is slowest in diagram .
f. The arrangement of particular is farther apart in diagram .
g. The particles fill the volume of the container in diagram .
h. If the water in diagram 2 has a mass of 19 g and a temperature of 45 °C, how much heat, in kilojoules, is removed to cool the liquid to 0 °C?

An energy bar contains carbohydrate, fat, and protein.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Study Guide And Selected Solutions Manual For Chemistry Format: Paperback
- (2 Pts) Draw correct Lewis structures for two different molecules that have C3H6 as theirchemical formulaarrow_forwardSynthesize the following:arrow_forwardDid you report your data to the correct number of significant figures? Temperature of cold water (°C) 4.0 Temperature of hot water ("C) 87.0 Volume of cold water (mL) 94.0 Volume of hot water (mL) 78.0 Final temperature after mixing ("C) 41.0 Mass of cold water (g) 94.0 Mass of hot water (g) 78.0 Calorimeter constant (J/°C) 12.44 How to calculate the calorimeter constantarrow_forward
- please add appropriate arrows and tell me in detail where to add which or draw itarrow_forwardPart 1. Draw monomer units of the following products and draw their reaction mechanism (with arrow pushing) Temporary cross-linked polymer Using: 4% polyvinyl alcohol+ methyl red + 4% sodium boratearrow_forwardcan you please answer both these questions and draw the neccesaryarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





