
Concept explainers
(a)
Interpretation:
The Lewis structure for the compound
(a)
Explanation of Solution
The given compound is
Step.1 Draw a skeletal structure of the molecule, arranging the atoms in their most probable order.
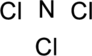
Step.2 Determine the number of valence electrons on each atom and add them to arrive at the total for the compound.
Step.3 Use the electron pairs to connect the central atom, nitrogen to each chlorine atom with a single bond.
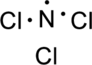
Step.4. Distribute the electrons around the terminal atoms in pairs:
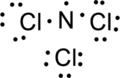
Step.5 Lewis structure of
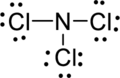
(b)
Interpretation:
The Lewis structure for the compound
(b)
Explanation of Solution
The given compound is
Step.1 Draw a skeletal structure of the molecule, arranging the atoms in their most probable order.
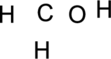
Step.2 Determine the number of valence electrons on each atom and add them to arrive at the total for the compound.
Step.3 Use the electron pairs to connect the central atom, carbon to each hydrogen and oxygen atom with a single bond.
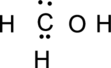
Step.4. Distribute the electrons around the terminal atoms in pairs:
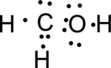
Step.5 Lewis structure of
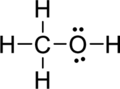
(c)
Interpretation:
The Lewis structure for the compound
(c)
Explanation of Solution
The given compound is
Step.1 Draw a skeletal structure of the molecule, arranging the atoms in their most probable order.
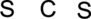
Step.2 Determine the number of valence electrons on each atom and add them to arrive at the total for the compound.
Step.3 Use the electron pairs to connect the central atom, carbon to each hydrogen and oxygen atom with a single bond.
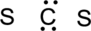
Step.4. Distribute the electrons around the terminal atoms in pairs:
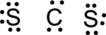
Step.5 Lewis structure of
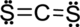
(d)
Interpretation:
The Lewis structure for the compound
(d)
Explanation of Solution
The given compound is
Step.1 Draw a skeletal structure of the molecule, arranging the atoms in their most probable order.
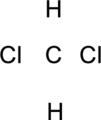
Step.2 Determine the number of valence electrons on each atom and add them to arrive at the total for the compound.
Step.3 Use the electron pairs to connect the central atom, carbon to each hydrogen and oxygen atom with a single bond.
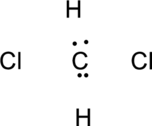
Step.4. Distribute the electrons around the terminal atoms in pairs:
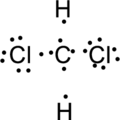
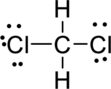
Step.5 Lewis structure of
Want to see more full solutions like this?
Chapter 3 Solutions
GENERAL ORGANIC+BIOCHEM.-ACCESS>CUSTOM<
- Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. O CH3CH2NH2, TSOH Select to Draw >arrow_forwardPredict the major organic product(s) for the following reaction.arrow_forwardPredict the major organic product(s) for the following reactions.arrow_forward
- Provide the complete mechanism for the reactions below. You must include appropriate arrows,intermediates, and formal charges.arrow_forwardIndicate the products obtained by reacting fluorobenzene with a sulfonitric mixture.arrow_forwardIf I have 1-bromopropene, to obtain compound A, I have to add NaOH and another compound. Indicate which compound that would be. C6H5 CH3arrow_forward
- If I have 1-bromopropene and I want to obtain (1,1-dipropoxyethyl)benzene, indicate the compound that I should add in addition to NaOH.arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. Ο HSCH2CH2CH2SH, BF3 Select to Draw I Submitarrow_forwardFeedback (7/10) Draw the major product of this reaction. Ignore inorganic byproducts. Assume that the water side product is continuously removed to drive the reaction toward products. Incorrect, 3 attempts remaining Ο (CH3CH2)2NH, TSOH Select to Draw V N. 87% Retryarrow_forward
- If I want to obtain (1,1-dipropoxyethyl)benzene from 1-bromopropene, indicate the product that I have to add in addition to NaOH.arrow_forwardIndicate the products obtained when fluorobenzene reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forwardIndicate the products obtained when chlorobenzene acid reacts with a sulfonitric acid mixture (HNO3 + H2SO4). Indicate the majority if necessary.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





