
(a)
Interpretation:
The symbol and name for the element contains only two
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their
Answer to Problem 3.28E
The name of the element that contains only two
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains only two
The name of the element that contains only two
(b)
Interpretation:
The symbol and name for the element contains an unpaired
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the element that contains an unpaired
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains an unpaired
The name of the element that contains an unpaired
(c)
Interpretation:
The symbol and name for the element contains two unpaired
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the elements that contain two unpaired
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains two unpaired
The element having electronic configuration
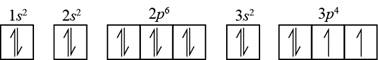
Figure 1
The element having electronic configuration
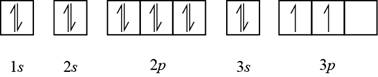
Figure 2
Hence, sulfur and silicon elements contain two unpaired
The name of the elements that contain two unpaired
(d)
Interpretation:
The symbol and name for the element contains three
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the element that contains three
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that three
The name of the element that contains three
(e)
Interpretation:
The symbol and name for the element contains three unpaired
Concept introduction:
The elements in a modern periodic table are arranged in increasing order of their atomic number. In the modern periodic table, the horizontal rows are known as periods and vertical columns are known as groups. There are
Answer to Problem 3.28E
The name of the element that contains three unpaired
Explanation of Solution
Electronic configuration tells about the arrangement of the electrons in each subshell and each orbit of an atom.
The electronic configuration for an element that contains three unpaired
The element having electronic configuration
![]()
Figure 3
The element having electronic configuration
![]()
Figure 4
Hence, vanadium and cobalt elements contain three unpaired
The name of the element that contains three unpaired
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry For Today: General, Organic, And Biochemistry, Loose-leaf Version
- If some molecules in an excited state collide with other molecules in a ground state, this process1. can occur in solution and in the gas phase.2. can be treated as a bimolecular process.3. always results in collisional deactivation.4. does not compete with any other process.arrow_forwardRadiation of frequency v is incident on molecules in their ground state. The expected outcome is that1. the molecules do not change their state.2. the molecules transition to an excited state.3. the molecules undergo a secondary process.4. collisional deactivation occurs.arrow_forwardPredict the major product of the following reaction and then draw a curved arrow mechanism for its formation. Part: 0/2 Part 1 of 2 H₂SO heat : OH 90 Draw the structure of the major product. Click and drag to start drawing a structure. 3arrow_forward
- Draw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all electrons that are necessary to the mechanism and all nonzero formal charges. C Ö-H H + -S-OH .0. Add/Remove step X टे Click and drag to start drawing a structure.arrow_forwardDraw a curved arrow mechanism for its formation. You may need to re-draw structures to show certain bonds. Ensure that HSO is used as the base to deprotonate the ẞ carbon when necessary. C HO : OH HO: OH =s = + 1 Add/Remove step X Click and drag to start drawing a structure.arrow_forwardWhich of the following could 1,2-ethanediol be directly synthesized from? OH HO О 0 0. O ?arrow_forward
- Design a synthesis of 1,2-diethoxyethane from an alkene. Select the single best answer for each part. Part: 0/3 Part 1 of 3 Which of the following could 1,2-diethoxyethane be directly synthesized from? O HO 0 HO.... OH HO HO × 5 > ?arrow_forwardDraw the skeletal structure of the major organic product of each step of the reaction sequence. Part: 0/2 Part 1 of 2 Part: 1/2 Part 2 of 2 Continue OH NaH Na Na Br + Click and drag to start drawing a structure. X : X G : Garrow_forwardpleasearrow_forward
- please help me please pleasearrow_forwardUsing reaction free energy to predict equilibrium composition Consider the following equilibrium: N2 (g) + 3H2 (g) = 2NH3 (g) AG⁰ = -34. KJ Now suppose a reaction vessel is filled with 8.06 atm of nitrogen (N2) and 2.58 atm of ammonia (NH3) at 106. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of N2 tend to rise or fall? ☐ x10 fall Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N2 will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of N2 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm ☑ 5 00. 18 Ararrow_forwardi need help with the followingarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





