
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
8th Edition
ISBN: 9781305582453
Author: Brown
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 3.24P
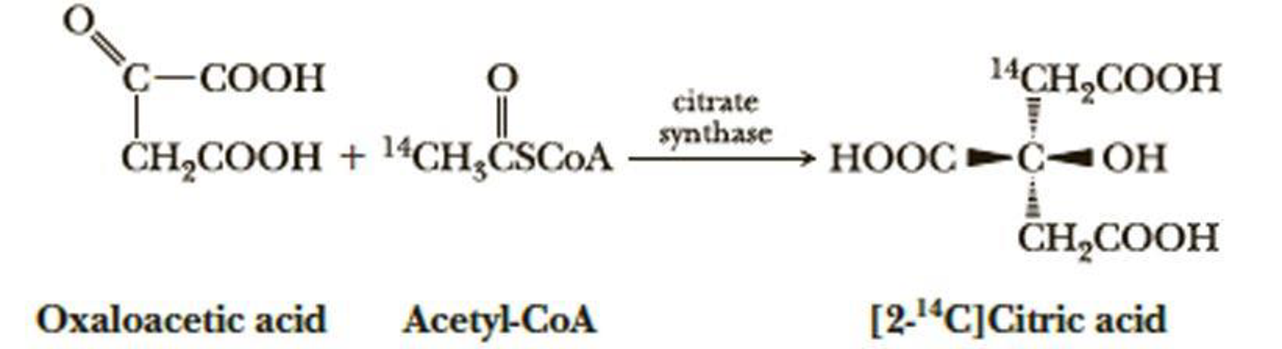
When oxaloacetic acid and acetyl-coenzyme A (acetyl-CoA) labeled with radioactive carbon-14 in position 2 are incubated with citrate synthase, an enzyme of the tricarboxylic acid cycle, only the following enantiomer of [2-l4C]citric acid is formed stereoselectively. Note that citric acid containing only 12C is achiral. Assign an R or S configuration to this enantiomer of [2-14C]citric acid. (Note: Carbon-14 has a higher priority than carbon-12.)
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
oalmitic acid is a 16 carbon acid. In a balanced equation, the products of the sponification of tripalmitin (glyceryl tripalmitate are blank.
Write the esterification reaction mechanism of salicylic acid and acetic acid to produce aspirin (acetylsalicylic acid). Note: salicylic acid will act as the alcohol
What type of interaction would you expect between the following R groups in the tertiary
structure of a protein?
O
-CH2-CO and -CH2-CH2-CH2-CH2-NH3+
a. disulfide bonds
b. salt bridges
c. hydrogen bonds
HO
abios vist anisinoo tedt bigil s ai loistaslor sale! 10 OUT
d. hydrophobic interactions
e. peptide bonds
Chapter 3 Solutions
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
Ch. 3.2 - Prob. 3.1PCh. 3.3 - Assign priorities to the groups in each set. (a)...Ch. 3.3 - Prob. 3.3PCh. 3.4 - Following are stereorepresentations for the four...Ch. 3.4 - Prob. 3.5PCh. 3.4 - Prob. 3.6PCh. 3.5 - How many stereoisomers exist for...Ch. 3.5 - How many stereoisomers exist for...Ch. 3.7 - Prob. 3.9PCh. 3.7 - Prob. 3.10P
Ch. 3.8 - If the side chain of the amino add is a methyl...Ch. 3.8 - Prob. BQCh. 3.8 - The amino acids cysteine and serine are shown....Ch. 3.8 - Prob. DQCh. 3.8 - As stated, proteins are stereochemically pure...Ch. 3.8 - As stated, proteins are stereochemically pure...Ch. 3 - Prob. 3.11PCh. 3 - One reason we can be sure that sp3-hybridized...Ch. 3 - Which compounds contain chiral centers? (a)...Ch. 3 - Prob. 3.15PCh. 3 - Prob. 3.16PCh. 3 - Prob. 3.17PCh. 3 - Mark each chiral center in the following molecules...Ch. 3 - Prob. 3.19PCh. 3 - Assign priorities to the groups in each set. (a) H...Ch. 3 - Following are structural formulas for the...Ch. 3 - Following is a staggered conformation for one of...Ch. 3 - Prob. 3.23PCh. 3 - When oxaloacetic acid and acetyl-coenzyme A...Ch. 3 - Prob. 3.25PCh. 3 - Mark each chiral center in the following molecules...Ch. 3 - Prob. 3.27PCh. 3 - Prob. 3.28PCh. 3 - Prob. 3.29PCh. 3 - Prob. 3.30PCh. 3 - Which of the following are meso compounds?Ch. 3 - Prob. 3.32PCh. 3 - Prob. 3.33PCh. 3 - Which of the following compounds are chiral?...Ch. 3 - Prob. 3.35PCh. 3 - Prob. 3.36PCh. 3 - Prob. 3.37PCh. 3 - The chiral catalyst (R)-BINAP-Ru is used to...Ch. 3 - Prob. 3.39P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 4. True or false: This skeletal structure represents a saturated fatty acid. Ini to 0 fale) me OH faistong starrow_forwardBy malonic or acetylacetic synthesis, synthesize 5-Methyl-2-hexanone (with the formulas of the compounds).arrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' by filling in all the empty green boxes *The values are all provided in the first photo attached*arrow_forward
- Draw the formula for 3-chlorobenzoic acetic anhydride.arrow_forwardBy malonic or acetylacetic synthesis, synthesize 2-methylbutanoic acid (indicate the formulas of the compounds).arrow_forwardObtain 2-methylbutanoic acid by malonic or acetylacetic synthesis (indicate the formulas of the compounds involved).arrow_forward
- EFFICIENTS SAMPLE READINGS CONCENTRATIONS Pigiadient) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) TOMATO SAUCE (REGULAR) TOMATO (REDUCED SALT) 58 6.274 3.898 301.7 151.2 14150 5.277 3.865 348.9 254.8 B 5.136 3.639 193.7 85.9 605 4.655 3.041 308.6 199.6 05 5.135 3.664 339.5 241.4 0139 4.676 3.662 160.6 87.6 90148 5.086 3.677 337.7 242.5 0092 6.348 3.775 464.7 186.4 PART3 5.081 3.908 223.5 155.8 5.558 3.861 370.5 257.1 4.922 3.66 326.6 242.9 4.752 3.641 327.5 253.3 50 5.018 3.815 336.1 256.0 84 4.959 3.605 317.9 216.6 38 4.96 3.652 203.8 108.7 $3 5.052 3.664 329.8 239.0 17 5.043 3.767 221.9 149.7 052 5.058 3.614 331.7 236.4 5.051 4.005 211.7 152.1 62 5.047 3.637 309.6 222.7 5.298 3.977 223.4 148.7 5.38 4.24 353.7 278.2 5 5.033 4.044 334.6 268.7 995 4.706 3.621 305.6 234.4 04 4.816 3.728 340.0 262.7 16 4.828 4.496 304.3 283.2 0.011 4.993 3.865 244.7 143.6 AVERAGE STDEV COUNT 95% CI Confidence Interval (mmol/L) [Na+] (mg/100 mL) 95% Na+ Confidence Interval (mg/100 mL)arrow_forwardIf we have two compounds: acetone (CH₃COCH₃) and acetic acid (CH₃COOH), applying heat to them produces an aldol condensation of the two compounds. If this is correct, draw the formula for the final product.arrow_forwardIf we have two compounds: acetone (CH3COCH3) and acetic acid (CH3COOH); if we apply heat (A), what product(s) are obtained?arrow_forward
- QUESTION: Fill out the answers to the empty green boxes attached in the image. *Ensure you all incorporate all 27 values (per column)*arrow_forwardYou need to make a buffer by dissolving benzoic acid and sodium benzoate in water. What is the mass of benzoic acid that you would weigh out, in mg, to create 50 mL of a buffer at pH = 4.7 that will change pH no more than 0.10 units with the addition of 0.001 moles of acid or base? Enter just the answer without the units (mg) - just the number will do!arrow_forwardDraw the formula for 3-isopropylcyclopentane-1-carbonyl chloride.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License