
Concept explainers
Determine the following information for an atom whose complete chemical symbol is
- a. The total number of subatomic particles present
- b. The total number of subatomic particles present in the nucleus of the atom
- c. The total number of nucleons present
- d. The total charge (including sign) associated with the nucleus of the atom
(a)
Interpretation:
The total number of subatomic particles present in
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
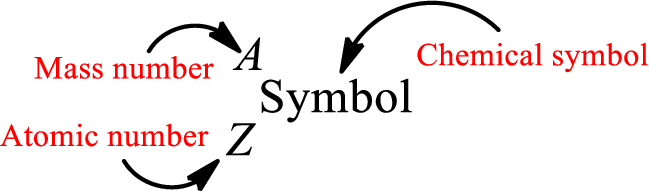
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given chemical symbol is
The total subatomic particles present in
(b)
Interpretation:
The total number of subatomic particles present nucleus of
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
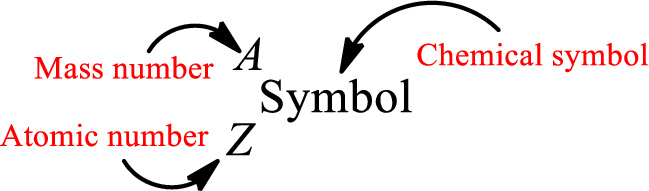
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given chemical symbol is
The total subatomic particles present in nucleus of
(c)
Interpretation:
The total number nucleons present in
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
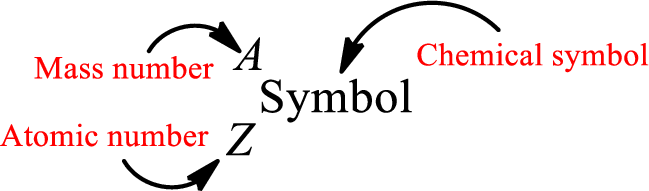
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given chemical symbol is
The total number of nucleons present in
(d)
Interpretation:
The total charge that is associated with nucleus of
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
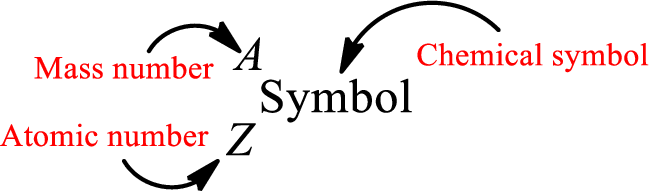
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
Explanation of Solution
Given chemical symbol is
The total charge associated with the nucleus of
Want to see more full solutions like this?
Chapter 3 Solutions
EBK GENERAL, ORGANIC, AND BIOLOGICAL CH
- Not part of a graded assignment, from a past midtermarrow_forwardNoggin mutation: The mouse, one of the phenotypic consequences of Noggin mutationis mispatterning of the spinal cord, in the posterior region of the mouse embryo, suchthat in the hindlimb region the more ventral fates are lost, and the dorsal Pax3 domain isexpanded. (this experiment is not in the lectures).a. Hypothesis for why: What would be your hypothesis for why the ventral fatesare lost and dorsal fates expanded? Include in your answer the words notochord,BMP, SHH and either (or both of) surface ectoderm or lateral plate mesodermarrow_forwardNot part of a graded assignment, from a past midtermarrow_forward
- Explain in a flowcharts organazing the words down below: genetics Chromosomes Inheritance DNA & Genes Mutations Proteinsarrow_forwardplease helparrow_forwardWhat does the heavy dark line along collecting duct tell us about water reabsorption in this individual at this time? What does the heavy dark line along collecting duct tell us about ADH secretion in this individual at this time?arrow_forward





