
DRAW IT Ø Label a hydrogen bond and a polar covalent bond in the diagram of live water molecules. Is a hydrogen bond a covalent bond? Explain.
To label: The hydrogen bond and covalent bonds in water molecules.
Introduction:
Water is a polar molecule consisting of an oxygen atom and two hydrogen atoms.
Explanation of Solution
Water (H2O) consists of an oxygen atom and two hydrogen atoms. Hydrogen and oxygen share their valence electrons to form a strong bond that known as a covalent bond (Fig.1). As oxygen is more electronegative than hydrogen, the shared electrons in the H-O bond tend to be pulled towards the oxygen atom. There are two regions of partial negative charge on oxygen and a partial positive charge on each hydrogen atom. Thus, the covalent bond between H-O in the water molecule is a polar bond.
Water is a polar molecule due to the difference in electronegativity between O and H atoms. The partial negatively charged oxygen atom of a water molecule is attracted to the partial positively charged hydrogen atom of an adjacent water molecule (Fig.1). This forms the hydrogen bond among different water molecules.
Pictorial representation:
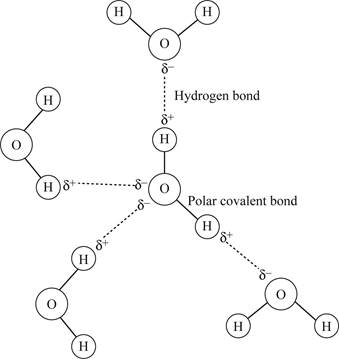
Fig. 1 Interactions among water molecules
To explain: The hydrogen bonds are not covalent bonds.
Introduction: Covalent bonds are formed by the sharing of valence electrons between two atoms. Hydrogen bonds are formed due to the attraction between two adjacent atoms due to partial charges.
Explanation of Solution
Covalent bonds are strong bonds, formed by the sharing of electrons between atoms. Hydrogen bonds do not involve the sharing of valence electrons. They are formed on the basis of attraction due to partial charges on neighboring atoms, and are hence very weak bonds. Therefore, a hydrogen bond is not a covalent bond.
Want to see more full solutions like this?
Chapter 3 Solutions
Campbell Biology Plus Masteringbiology
Additional Science Textbook Solutions
Biological Science (6th Edition)
Chemistry & Chemical Reactivity
Organic Chemistry
Microbiology Fundamentals: A Clinical Approach
Human Physiology: An Integrated Approach (8th Edition)
- Molecular Biology Please help with question. Thank you in advance. Discuss, compare and contrast the structure of promoters inprokaryotes and eukaryotes.arrow_forwardMolecular Biology Please help with question. Thank you You are studying the expression of the lac operon. You have isolated mutants as described below. In the absence of glucose, explain/describe what would happen, for each mutant, to the expression of the lac operon when you add lactose AND what would happen when the bacteria has used up all of the lactose (if the mutant is able to use lactose).1. Mutations in the lac repressor gene that would prevent the binding of lactose2. Mutations in the lac repressor gene that would prevent release of lactose once lactose hadbound3. Normally the lac repressor gene is located next to (a few hundred base pairs) and upstreamfrom the lac operon. Mutations in the lac repressor gene that move the lac repressor gene 100,000base pairs downstream.4. Mutations in the lac operator that would prevent binding of lac repressorarrow_forwardYou have returned to college to become a phylogeneticist. One of the first things you wish to do is determine how mammals, birds, and reptiles are related. Like any good scientist, you need to consider all available data objectively and without a preconceived “correct” answer. In pursuit of that, you should produce a phylogenetic tree based only on morphological features that show birds and mammals are more closely related. You will then produce a totally different tree, also using morphological features, that shows birds and reptiles are more closely related. Do not forget to include all three groups in both your trees. Based solely off the trees you produce, which relationship would you consider the more likely and why? Once you have answered that question, provide a brief summary of the “modern” understanding of the relationship between these three groups.arrow_forward
- true or false, the reason geckos can walk on walls is hydrogen bonding between their foot pads and the moisture on the wall.arrow_forwardBiology laboratory problem Please help. thank you You have 20 ul of DNA solution and 6X DNA loading buffer solution. You have to mix your DNA solution and DNA loading buffer before load DNA in an agarose gel. The concentration of the DNA loading buffer must be 1X in the DNA and DNA-loading buffer mixture after you mix them. For that, I will add _____ ul of 6X loading buffer to the 20 ul DNA solution.arrow_forwardBiology lab problem To make 20 ul of 5 mM MgCl2 solution using 50 mM MgCl2 stock solution and distilled water, I will mix ________ ul of 50 mM MgCl2 solution and ________ ul of distilled water. Please help . Thank youarrow_forward
- Biology Please help. Thank you. Biology laboratory question You need 50 ml of 1% (w/v) agarose gel. Agarose is a powder. How would you make it? You can ignore the volume of agarose powder. Don't forget the unit.TBE buffer is used to make an agarose gel, not distilled water. I will add _______ of agarose powder into 50 ml of distilled water (final 50 ml).arrow_forwardAn urgent care center experienced the average patient admissions shown in the Table below during the weeks from the first week of December through the second week of April. Week Average Daily Admissions 1-Dec 11 2-Dec 14 3-Dec 17 4-Dec 15 1-Jan 12 2-Jan 11 3-Jan 9 4-Jan 9 1-Feb 12 2-Feb 8 3-Feb 13 4-Feb 11 1-Mar 15 2-Mar 17 3-Mar 14 4-Mar 19 5-Mar 13 1-Apr 17 2-Apr 13 Forecast admissions for the periods from the first week of December through the second week of April. Compare the forecast admissions to the actual admissions; What do you conclude?arrow_forwardAnalyze the effectiveness of the a drug treatment program based on the needs of 18-65 year olds who are in need of treatment by critically describing 4 things in the program is doing effectively and 4 things the program needs some improvement.arrow_forward
- I have the first half finished... just need the bottom half.arrow_forward13. Practice Calculations: 3 colonies were suspended in the following dilution series and then a viable plate count and microscope count was performed. Calculate IDF's, TDF's and then calculate the CFU/mL in each tube by both methods. Finally calculate the cells in 1 colony by both methods. Show all of your calculations in the space provided on the following pages. 3 colonies 56 cells 10 μL 10 μL 100 μL 500 με m OS A B D 5.0 mL 990 με 990 με 900 με 500 μL EN 2 100 με 100 μL 118 colonies 12 coloniesarrow_forwardDescribe and give a specific example of how successionary stage is related to species diversity?arrow_forward

 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College
Anatomy & PhysiologyBiologyISBN:9781938168130Author:Kelly A. Young, James A. Wise, Peter DeSaix, Dean H. Kruse, Brandon Poe, Eddie Johnson, Jody E. Johnson, Oksana Korol, J. Gordon Betts, Mark WomblePublisher:OpenStax College Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology: The Unity and Diversity of Life (MindTap...BiologyISBN:9781305073951Author:Cecie Starr, Ralph Taggart, Christine Evers, Lisa StarrPublisher:Cengage Learning





