
Concept explainers
Give the IUPAC names of each of the following:
(a) (b) (c)
Interpretation:
IUPAC names of the given structures are to be determined.
Concept introduction:
When writing the IUPAC name, the longest continuous carbon chain is first determined. The parent chain is numbered such that the substituents get the lowest numbers.
The location of each substituent group is designated by an appropriate number and name. Prefixes are used if more than one substituents of the same type are present. The substituents are written in alphabetical order.
A cyclic ring hydrocarbon is designated by the prefix cyclo- which appears in front of the base name.
Answer to Problem 20P
Solution:
Explanation of Solution
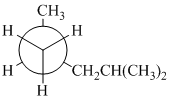
(a)
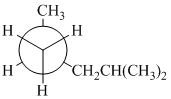
In the given Newman projection, the carbon atom at the front is a
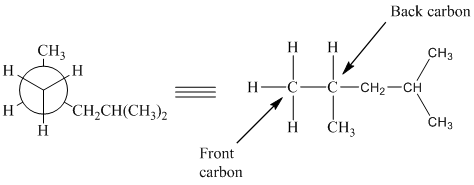
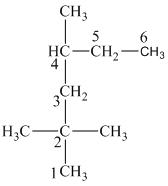
In this structure, the longest chain contains five carbon atoms, so the parent alkane is pentane. There are two methyl groups attached to this parent chain. The pentane chain is numbered such that these methyl groups get the lowest numbers.
The two methyl groups are attached to the carbon atoms
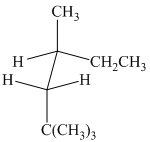
(b)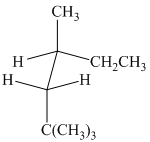
The given sawhorse projection is converted to a skeletal structure as follows:
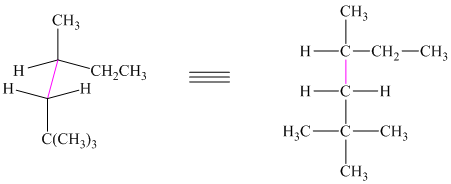
In this structure, the longest chain contains six carbon atoms, so the parent alkane is hexane. There are three methyl groups attached to the hexane chain. The chain is numbered such that the carbon atoms attached to these three methyl groups get the lowest numbers.
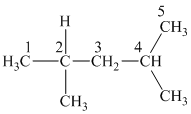
Two methyl groups are attached to
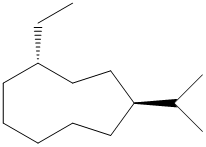
(c)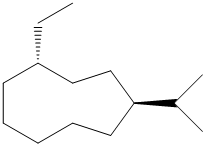
In the given structure, there are nine carbon atoms in the ring. So the name of parent alkane is cyclononane. The ring is numbered such that the two substituents get the lowest numbers.
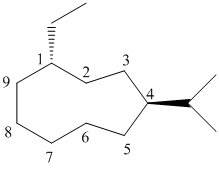
There is an ethyl group and an isopropyl group attached to the cyclononane ring. These two groups are trans to each other. Hence the ring is numbered such that the ethyl group gets the lower number since it comes first alphabetically. The IUPAC name of the compound is
Want to see more full solutions like this?
Chapter 3 Solutions
ORGANIC CHEMISTRY (LOOSELEAF)-PACKAGE
- Polar solutes are most likely to dissolve into _____, and _____ are most likely to dissolve into nonpolar solvents. A. nonpolar solutes; polar solvents B. nonpolar solvents; polar solvents C. polar solvents; nonpolar solutes D. polar solutes; nonpolar solventsarrow_forwardDeducing the Peactants Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Xarrow_forwardDraw all 8 stereoisomers, circling each pair of enantiomer(s)/ mirror image compound(s)arrow_forward
- Bookmarks Profiles Tab Window Help Chemical Formula - Aktiv Che X + → C 11 a app.aktiv.com Google Chrome isn't your default browser Set as default Question 12 of 16 Q Fri Feb 2 Verify it's you New Chrome availabl- Write the balanced molecular chemical equation for the reaction in aqueous solution for mercury(I) nitrate and chromium(VI) sulfate. If no reaction occurs, simply write only NR. Be sure to include the proper phases for all species within the reaction. 3 Hg(NO3)2(aq) + Cг2(SO4)3(aq) → 3 Hg₂SO (s) + 2 Cr(NO3), (aq) ean Ui mate co ence an climate bility inc ulnerabili women, main critic CLIMATE-INI ernational + 10 O 2 W FEB 1 + 4- 3- 2- 2 2 ( 3 4 NS 28 2 ty 56 + 2+ 3+ 4+ 7 8 9 0 5 (s) (1) Ch O 8 9 (g) (aq) Hg NR CI Cr x H₂O A 80 Q A DII A F2 F3 FA F5 F6 F7 F8 F9 #3 EA $ do 50 % 6 CO & 7 E R T Y U 8 ( 9 0 F10 34 F11 川 F12 Subr + delete 0 { P }arrow_forwardDeducing the reactants of a Diels-Alder reaction n the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. >arrow_forwardPredict the major products of the following organic reaction: + Some important notes: A ? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure.arrow_forward
- if the answer is no reaction than state that and please hand draw!arrow_forward"I have written solutions in text form, but I need experts to rewrite them in handwriting from A to Z, exactly as I have written, without any changes."arrow_forwardDon't used hand raiting and don't used Ai solutionarrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

