
Draw the four water molecules that can hydrogen-bond to this water molecule. Label the bonds and the partial negative and positive charges that account for the formation of these hydrogen bonds.
To draw: The four water molecules that can form hydrogen-bond to the given water molecule and label the bonds and the partial negative and positive charges that account for the formation of these hydrogen bonds.
Introduction: A water molecule is shaped like a ‘V’ that consists of an oxygen atom and two hydrogen atoms. Oxygen is more electronegative than hydrogen. Thus, the shared electrons in the O-H covalent bond are more attracted by the oxygen atom.
Answer to Problem 1IQ
Pictorial representation: Fig.1 represents the four water molecules that can form hydrogen-bond for the given water molecule and label the bonds and the partial negative and positive charges that account for the formation of these hydrogen bonds.
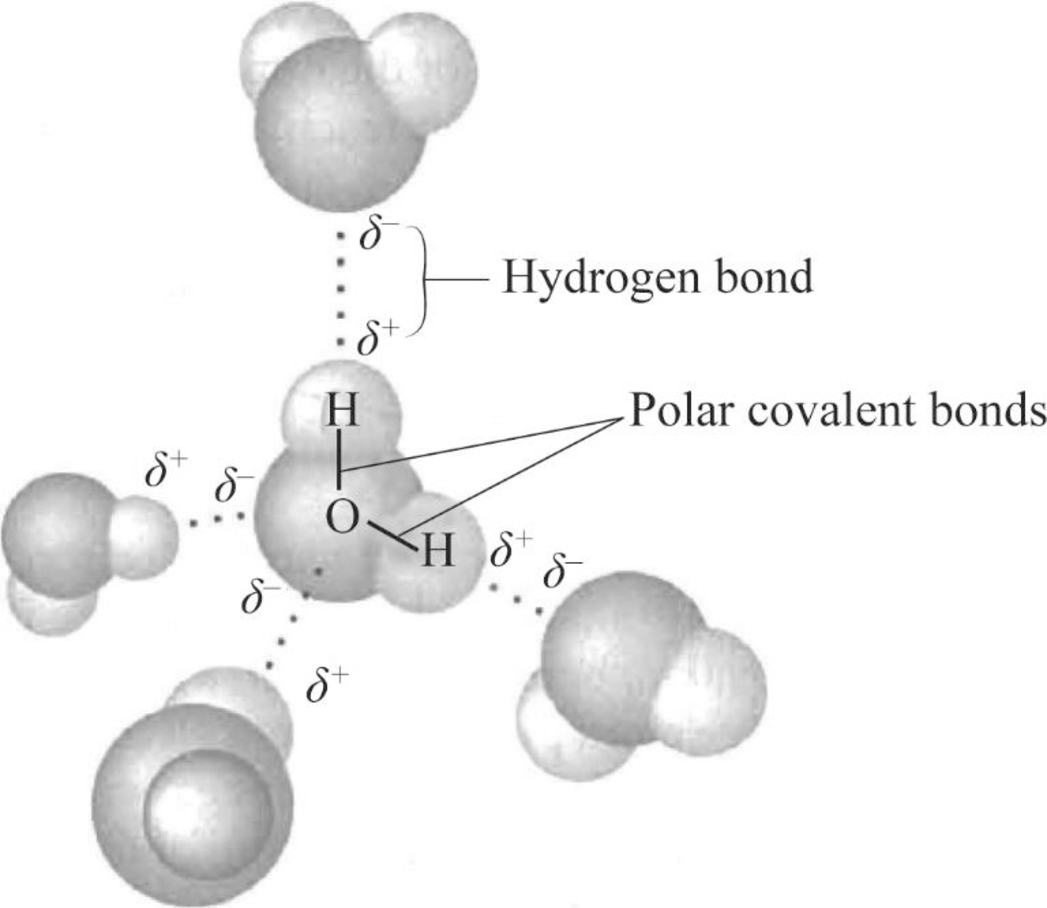
Fig.1: Four water molecules bind with one water molecule
Explanation of Solution
Electronegativity is the tendency of an atom in a molecule to attract electrons shared in a covalent bond. If one atom is more electronegative, the electrons would be more on the side of that atom. Oxygen is more electronegative than hydrogen. In a water molecule, there are two polar covalent bonds between oxygen and hydrogen. Hence, there are two regions of partial negative charge on oxygen and a partial positive charge on each hydrogen atom.
The partial negatively charged oxygen atom of a water molecule is attracted to the partial positively charged hydrogen of the other water molecule. As per Fig.1, atoms on the partial charged regions can bind to oppositely charged regions on other adjoining water molecules. There are four regions of partial charge in a water molecule. Thus, the central water molecule can form a hydrogen bonds with four other water molecules.
Want to see more full solutions like this?
Chapter 3 Solutions
Study Guide for Campbell Biology
- Outline the negative feedback loop that allows us to maintain a healthy water concentration in our blood. You may use diagram if you wisharrow_forwardGive examples of fat soluble and non-fat soluble hormonesarrow_forwardJust click view full document and register so you can see the whole document. how do i access this. following from the previous question; https://www.bartleby.com/questions-and-answers/hi-hi-with-this-unit-assessment-psy4406-tp4-report-assessment-material-case-stydu-ms-alecia-moore.-o/5e09906a-5101-4297-a8f7-49449b0bb5a7. on Google this image comes up and i have signed/ payed for the service and unable to access the full document. are you able to copy and past to this response. please see the screenshot from google page. unfortunality its not allowing me attch the image can you please show me the mathmetic calculation/ workout for the reult sectionarrow_forward
- Skryf n kortkuns van die Egyptians pyramids vertel ñ story. Maximum 500 woordearrow_forward1.)What cross will result in half homozygous dominant offspring and half heterozygous offspring? 2.) What cross will result in all heterozygous offspring?arrow_forward1.Steroids like testosterone and estrogen are nonpolar and large (~18 carbons). Steroids diffuse through membranes without transporters. Compare and contrast the remaining substances and circle the three substances that can diffuse through a membrane the fastest, without a transporter. Put a square around the other substance that can also diffuse through a membrane (1000x slower but also without a transporter). Molecule Steroid H+ CO₂ Glucose (C6H12O6) H₂O Na+ N₂ Size (Small/Big) Big Nonpolar/Polar/ Nonpolar lonizedarrow_forward
- what are the answer from the bookarrow_forwardwhat is lung cancer why plants removes liquid water intead water vapoursarrow_forward*Example 2: Tracing the path of an autosomal dominant trait Trait: Neurofibromatosis Forms of the trait: The dominant form is neurofibromatosis, caused by the production of an abnormal form of the protein neurofibromin. Affected individuals show spots of abnormal skin pigmentation and non-cancerous tumors that can interfere with the nervous system and cause blindness. Some tumors can convert to a cancerous form. i The recessive form is a normal protein - in other words, no neurofibromatosis.moovi A typical pedigree for a family that carries neurofibromatosis is shown below. Note that carriers are not indicated with half-colored shapes in this chart. Use the letter "N" to indicate the dominant neurofibromatosis allele, and the letter "n" for the normal allele. Nn nn nn 2 nn Nn A 3 N-arrow_forward
 Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
 Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning





