
Using Figure 3.1, determine
a. the number of grams of
b. the number of grams of water required to dissolve
c. the number of grams of water required to dissolve
d. the number of grams of water required to dissolve a mixture containing
(a)
Interpretation:
The number of grams of
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of
Explanation of Solution
The given illustration of the graph is shown below.
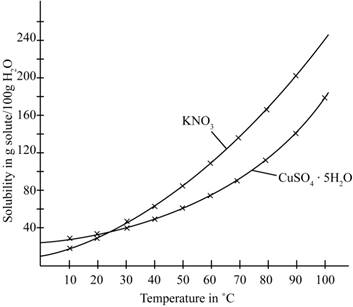
Figure 1
According to the above graph, the number of grams of
The number of grams of
(b)
Interpretation:
The number of grams of water required to dissolve
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of water required to dissolve
Explanation of Solution
The given illustration of the graph is shown below.
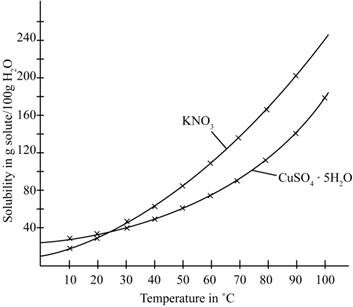
Figure 1
According to the above graph, the number of grams of
Thus, water required to dissolve
The number of grams of water required to dissolve
(c)
Interpretation:
The number of grams of water required to dissolve
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of water required to dissolve
Explanation of Solution
The given illustration of the graph is shown below.
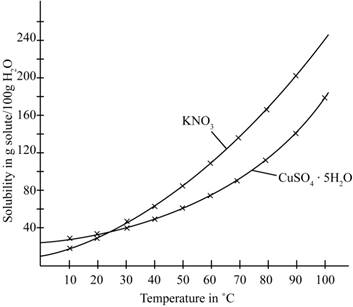
Figure 1
According to the above graph, the number of grams of
Thus, the water required to dissolve
The number of grams of water required to dissolve
(d)
Interpretation:
The number of grams of water required to dissolve a mixture containing
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of water required to dissolve a mixture containing
Explanation of Solution
The given illustration of the graph is shown below.
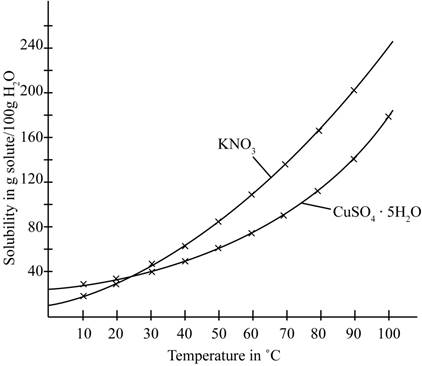
Figure 1
The amount of water required to dissolve
The amount of water required to dissolve
Therefore, the total amount of water required to dissolve a mixture containing
The number of grams of water required to dissolve a mixture containing
Want to see more full solutions like this?
Chapter 3 Solutions
Chemical Principles in the Laboratory
- Redraw the flowchartarrow_forwardredraw the flowchart with boxes and molecules written in themarrow_forwardPart I. a) Elucidate the structure of compound A using the following information. • mass spectrum: m+ = 102, m/2=57 312=29 • IR spectrum: 1002.5 % TRANSMITTANCE Ngg 50 40 30 20 90 80 70 60 MICRONS 5 8 9 10 12 13 14 15 16 19 1740 cm M 10 0 4000 3600 3200 2800 2400 2000 1800 1600 13 • CNMR 'H -NMR Peak 8 ppm (H) Integration multiplicity a 1.5 (3H) triplet b 1.3 1.5 (3H) triplet C 2.3 1 (2H) quartet d 4.1 1 (2H) quartet & ppm (c) 10 15 28 60 177 (C=0) b) Elucidate the structure of compound B using the following information 13C/DEPT NMR 150.9 MHz IIL 1400 WAVENUMBERS (CM-1) DEPT-90 DEPT-135 85 80 75 70 65 60 55 50 45 40 35 30 25 20 ppm 1200 1000 800 600 400arrow_forward
- • Part II. a) Elucidate The structure of compound c w/ molecular formula C10 11202 and the following data below: • IR spectra % TRANSMITTANCE 1002.5 90 80 70 60 50 40 30 20 10 0 4000 3600 3200 2800 2400 2000 1800 1600 • Information from 'HAMR MICRONS 8 9 10 11 14 15 16 19 25 1400 WAVENUMBERS (CM-1) 1200 1000 800 600 400 peak 8 ppm Integration multiplicity a 2.1 1.5 (3H) Singlet b 3.6 1 (2H) singlet с 3.8 1.5 (3H) Singlet d 6.8 1(2H) doublet 7.1 1(2H) doublet Information from 13C-nmR Normal carbon 29ppm Dept 135 Dept -90 + NO peak NO peak 50 ppm 55 ppm + NO peak 114 ppm t 126 ppm No peak NO peak 130 ppm t + 159 ppm No peak NO peak 207 ppm по реак NO peakarrow_forwardCould you redraw these and also explain how to solve them for me pleasarrow_forwardNonearrow_forward
- Draw the curved-arrow mechanism with the drawings of the molecules, not just abbreviations. -NO₂ Sn, HCl (aq) E D H (CH3CO)₂O -NH2 CH3arrow_forwardWhat is/are the product(s) of the following reaction? Select all that apply. * HI A B C OD OH A B OH D Carrow_forwardIn the image, the light blue sphere represents a mole of hydrogen atoms, the purple or teal spheres represent a mole of a conjugate base. A light blue sphere by itself is H+. Assuming there is 2.00 L of solution, answer the following: The Ka of the left & right solution is? The pH of the left & right solution is? The acid on the left & right is what kind of acid?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





