
Concept explainers
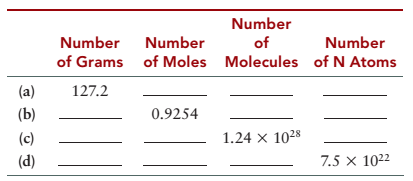
Complete the following table for TNT (trinitrotoluene), C7H5(NO2)3.
Interpretation:
The given table should be completed.
Concept introduction:
The number of moles of a substance is related to mass and molar mass as follows:
Here, m is mass and M is molar mass of the substance.
Also, according to Avogadro’s law in 1 mol of a substance there are
The density of a substance is related to mass and volume as follows:
Here, m is mass and V is volume.
Answer to Problem 13QAP
| Number of grams | Number of moles | Number of molecules | Number of N atoms | |
| (a) | |
|
|
|
| (b) | |
|
|
|
| (c) | |
|
|
|
| (d) | |
|
|
|
Explanation of Solution
The given compound is TNT (trinitrotoluene) with molecular formula
The molar mass of carbon, hydrogen, nitrogen and oxygen is 12 g/mol, 1 g/mol, 14 g/mol and 16 g/mol respectively.
Putting the values,
Step (a)
The mass of TNT is 127.2 g. The number of moles can be calculated as follows:
Putting the values,
Since, according to Avogadro’s law in 1 mol of a substance there are
Thus, number of molecules in 0.56 mol of TNT will be:
Thus, number of molecules of TNT is
Now, the molecular formula of TNT is
Thus, number of N atoms will be:
Therefore, number of N atoms is
Step (b)
The number of moles of TNT is
Putting the values,
The number of molecules of TNT can be calculated as follows:
Now, in 1 mol there are 3 nitrogen atoms. Thus, the number of N atoms will be 3 times the number of molecules of TNT.
Step (c)
The number of molecules of TNT is
Thus, number of N atoms in
According to Avogadro’s law, in mol there are
Since, molar mass of TNT is 227 g/mol thus, mass can be calculated as follows:
Step (d)
The number of N atoms is
Since, the number of N atoms is 3 times the number of TNT molecule. Thus, number of molecules of TNT will be:
According to Avogadro’s law, in mol there are
Since, molar mass of TNT is 227 g/mol thus, mass can be calculated as follows:
Therefore, the complete table will be as follows:
| Number of grams | Number of moles | Number of molecules | Number of N atoms | |
| (a) | |
|
|
|
| (b) | |
|
|
|
| (c) | |
|
|
|
| (d) | |
|
|
|
Want to see more full solutions like this?
Chapter 3 Solutions
Bundle: Chemistry: Principles and Reactions, 8th, Loose-Leaf + OWLv2, 1 term (6 months) Printed Access Card
- Nonearrow_forwardA complete tensile test was performed on a magnesium specimen of 12 mm diameter and 30 mm length, until breaking. The specimen is assumed to maintain a constant volume. Calculate the approximate value of the actual stress at breaking. TABLE. The tensile force F and the length of the specimen are represented for each L until breaking. F/N L/mm 0 30,0000 30,0296 5000 10000 30,0592 15000 30,0888 20000 30,15 25000 30,51 26500 30,90 27000 31,50 26500 32,10 25000 32,79arrow_forwardNonearrow_forward
- Differentiate between plastic deformation, elastic deformation, viscoelastic deformation and viscoplastic deformation.arrow_forward1.57 Draw all reasonable resonance structures for the following cation. Then draw the resonance hybrid.arrow_forwardFor the two questions below, draw the mechanism and form the major product.arrow_forward
- Indicate similarities and differences between natural, exchanged and pillared clays.arrow_forwardShow work. don't give Ai generated solutionarrow_forwardIn intercalation compounds, their sheets can be neutral or have a negative or positive charge, depending on the nature of the incorporated species and its structure. Is this statement correct?arrow_forward
- This thermodynamic cycle describes the formation of an ionic compound MX2 from a metal element M and nonmetal element X in their standard states. What is the lattice enthalpy of MX2 ? What is the enthalpy formation of MX2 ? Suppose both the heat of sublimation of M and the ionization enthalpy of M were smaller. Would MX2 be more stable? Or less? or impossible to tell without more information?arrow_forward7. Draw the mechanism to describe the following transformation: Note: This is a base catalyzed reaction. So, the last steps must make [OH]- OH [OH]¯ OH Heat Oarrow_forwardShow work with explanation...don't give Ai generated solutionarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





