
Concept explainers
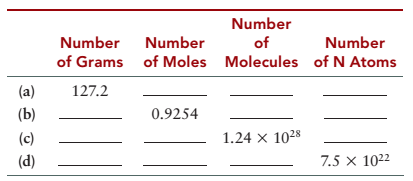
Complete the following table for TNT (trinitrotoluene), C7H5(NO2)3.
Interpretation:
The given table should be completed.
Concept introduction:
The number of moles of a substance is related to mass and molar mass as follows:
Here, m is mass and M is molar mass of the substance.
Also, according to Avogadro’s law in 1 mol of a substance there are
The density of a substance is related to mass and volume as follows:
Here, m is mass and V is volume.
Answer to Problem 13QAP
| Number of grams | Number of moles | Number of molecules | Number of N atoms | |
| (a) | |
|
|
|
| (b) | |
|
|
|
| (c) | |
|
|
|
| (d) | |
|
|
|
Explanation of Solution
The given compound is TNT (trinitrotoluene) with molecular formula
The molar mass of carbon, hydrogen, nitrogen and oxygen is 12 g/mol, 1 g/mol, 14 g/mol and 16 g/mol respectively.
Putting the values,
Step (a)
The mass of TNT is 127.2 g. The number of moles can be calculated as follows:
Putting the values,
Since, according to Avogadro’s law in 1 mol of a substance there are
Thus, number of molecules in 0.56 mol of TNT will be:
Thus, number of molecules of TNT is
Now, the molecular formula of TNT is
Thus, number of N atoms will be:
Therefore, number of N atoms is
Step (b)
The number of moles of TNT is
Putting the values,
The number of molecules of TNT can be calculated as follows:
Now, in 1 mol there are 3 nitrogen atoms. Thus, the number of N atoms will be 3 times the number of molecules of TNT.
Step (c)
The number of molecules of TNT is
Thus, number of N atoms in
According to Avogadro’s law, in mol there are
Since, molar mass of TNT is 227 g/mol thus, mass can be calculated as follows:
Step (d)
The number of N atoms is
Since, the number of N atoms is 3 times the number of TNT molecule. Thus, number of molecules of TNT will be:
According to Avogadro’s law, in mol there are
Since, molar mass of TNT is 227 g/mol thus, mass can be calculated as follows:
Therefore, the complete table will be as follows:
| Number of grams | Number of moles | Number of molecules | Number of N atoms | |
| (a) | |
|
|
|
| (b) | |
|
|
|
| (c) | |
|
|
|
| (d) | |
|
|
|
Want to see more full solutions like this?
Chapter 3 Solutions
EBK CHEMISTRY: PRINCIPLES AND REACTIONS
- Draw the structure of the product of the reaction given the IR and MS data. Spectral analysis of the product reveals: MS: M 150, M-15, M-43 CH.COCI AICI, IR: 3150-3000 cm, 2950-2850 cm and 1700 cmarrow_forwardPart II. Identify whether the two protons in blue are homotopic, enantiopic, diasteriotopic, or heterotopic. a) HO b) Bri H HH c) d) H H H Br 0arrow_forwardNonearrow_forward
- Choose the option that is decreasing from biggest to smallest. Group of answer choices: 100 m, 10000 mm, 100 cm, 100000 um, 10000000 nm 10000000 nm, 100000 um, 100 cm, 10000 mm, 100 m 10000000 nm, 100000 um, 10000 mm, 100 cm, 100 m 100 m, 100 cm, 10000 mm, 100000 um, 10000000 nmarrow_forwardQ1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forwardWhich is NOT the typical size of a bacteria? 1000 nm 0.001 mm 0.01 mm 1 umarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





