(a)
To determine:
Whether a H bond will be formed in a reaction between H3C-CH=O and H2O with the help of a figure.
Introduction:
(b)
To determine:
With the help of diagrams if the molecules Cl2, NH3 and CH4 will be polar or not.
Introduction:
When atoms combine to form molecules, they form chemical bonds by sharing, donating or accepting electrons. In case of sharing, the combining atoms may or may not share the electrons equally. If one of the atoms is more electronegative, it pulls the shared electron cloud towards itself and acquires negative charge. The other atom thus acquires positive charge. Such a molecule is called polar.
(c)
To determine:
The pH of a solution with a H+ concentration of 0.00001 M.
Introduction:
Dissociation of water results in generation of H+ and OH- ions. However, these ions are equal in number. Acids are compounds that release H+ in solution and bases are compounds that release OH- in solution. The pH scale expresses the concentration of H+ ions in a solution. pH less than 7 is indicative of acidic solution and pH above 7 indicates a basic (alkaline) solution. pH 7 is an indicator of a neutral solution.
(d)
To determine:
The pH of a solution with a OH- concentration of 0.00001 M.
Introduction:
Dissociation of water results in generation of H+ and OH- ions. However, these ions are equal in number. Acids are compounds that release H+ in solution and bases are compounds that release OH- in solution. The pH scale expresses the concentration of H+ ions in a solution. pH less than 7 is indicative of acidic solution and pH above 7 indicates a basic (alkaline) solution. pH 7 is an indicator of a neutral solution.
(e)
To determine:
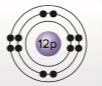
The kinds of bonds that magnesium can make based on the diagram of its atomic structure.
Introduction:
Atoms combine with other atoms by making chemical bonds with them. These bonds can be made by either sharing, donating or accepting electrons. The number of electrons in the outer-most orbital of an atom determine its tendency to form bonds. These electrons are called valence electrons. Chemical bonds are formed in a way that the outer-most orbital is completely filled with electrons.
(f)
To determine:
The kind of ion that Magnesium would make, depending on its valence.
Introduction:
Atoms combine with other atoms by making chemical bonds with them. These bonds can be made by either sharing, donating or accepting electrons. The number of electrons in the outer-most orbital of an atom determine its tendency to form bonds. These electrons are called valence electrons. Chemical bonds are formed in a way that the outer-most orbital is completely filled with electrons.
Explanation:
Looking at the atomic structure of Magnesium, it is observed that the 12 electrons are distributed is different orbital as 2, 8 and 2 electrons. The outer-most orbital has 2 electrons. Thus, Mg forms bonds by giving up its two valence electrons to atoms that may accept them. Thus, Mg loses 2 electrons to become positively charged ion Mg2+. Positively charged ions are also known as cations.
Conclusion:
Magnesium loses two electrons from its outer-most orbital to gain positive charge on its ion.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Foundations in Microbiology
- Biology lab problem To make 20 ul of 5 mM MgCl2 solution using 50 mM MgCl2 stock solution and distilled water, I will mix ________ ul of 50 mM MgCl2 solution and ________ ul of distilled water. Please help . Thank youarrow_forwardBiology Please help. Thank you. Biology laboratory question You need 50 ml of 1% (w/v) agarose gel. Agarose is a powder. How would you make it? You can ignore the volume of agarose powder. Don't forget the unit.TBE buffer is used to make an agarose gel, not distilled water. I will add _______ of agarose powder into 50 ml of distilled water (final 50 ml).arrow_forwardAn urgent care center experienced the average patient admissions shown in the Table below during the weeks from the first week of December through the second week of April. Week Average Daily Admissions 1-Dec 11 2-Dec 14 3-Dec 17 4-Dec 15 1-Jan 12 2-Jan 11 3-Jan 9 4-Jan 9 1-Feb 12 2-Feb 8 3-Feb 13 4-Feb 11 1-Mar 15 2-Mar 17 3-Mar 14 4-Mar 19 5-Mar 13 1-Apr 17 2-Apr 13 Forecast admissions for the periods from the first week of December through the second week of April. Compare the forecast admissions to the actual admissions; What do you conclude?arrow_forward
- Analyze the effectiveness of the a drug treatment program based on the needs of 18-65 year olds who are in need of treatment by critically describing 4 things in the program is doing effectively and 4 things the program needs some improvement.arrow_forwardI have the first half finished... just need the bottom half.arrow_forward13. Practice Calculations: 3 colonies were suspended in the following dilution series and then a viable plate count and microscope count was performed. Calculate IDF's, TDF's and then calculate the CFU/mL in each tube by both methods. Finally calculate the cells in 1 colony by both methods. Show all of your calculations in the space provided on the following pages. 3 colonies 56 cells 10 μL 10 μL 100 μL 500 με m OS A B D 5.0 mL 990 με 990 με 900 με 500 μL EN 2 100 με 100 μL 118 colonies 12 coloniesarrow_forward
- Describe and give a specific example of how successionary stage is related to species diversity?arrow_forwardExplain down bellow what happens to the cell in pictures not in words: Decreased pH in mitochondria Increased ATP Decreased pH in cytosol Increased hydrolysis Decreasing glycogen and triglycerides Increased MAP kinase activity Poor ion transport → For each one:→ What normally happens?→ What is wrong now?→ How does it mess up the cell?arrow_forward1.) Community Diversity: The brown and orange line represent two different plant communities. a. Which color represents the community with a higher species richness? b. Which color represents the community with a higher species evenness? Relative abundance 0.1 0.04 0.001 2 4 6 8 10 12 14 16 18 20 22 24 Rank abundance c. What is the maximum value of the Simpson's diversity index (remember, Simpson's index is D = p², Simpson's diversity index is 1-D)? d. If the Simpson's diversity index equals 1, what does that mean about the number of species and their relative abundance within community being assessed?arrow_forward
- 1.) Community Diversity: The brown and orange line represent two different plant communities. a. Which color represents the community with a higher species richness? b. Which color represents the community with a higher species evenness? Relative abundance 0.1 0.04 0.001 2 4 6 8 10 12 14 16 18 20 22 24 Rank abundance c. What is the maximum value of the Simpson's diversity index (remember, Simpson's index is D = p², Simpson's diversity index is 1-D)? d. If the Simpson's diversity index equals 1, what does that mean about the number of species and their relative abundance within community being assessed?arrow_forwardwhat measures can a mother to take to improve the produce of her to milk to her newborn baby ?arrow_forward1. Color the line that represents all ancestors of the Eastern white pine tree green (but only the ancestral line NOT shared with other organisms) 2. Oncle the last common ancestor of the Colorado blue spruce tree and Eastern white pine tree. 3. Put a box around the last common ancestor of the sugar maple tree and the dogwood tree. 4. Put a triangle around the last common ancestor of the red pine tree and the american holly bush. 5. Color the line that represents all ancestors of the Ponderosa pine tree red (including all shared ancestors). 6. Color the line that represents all ancestors of the American elm tree blue (including all shared ancestors). 7 Color the line that represents all ancestors of the Sabal palm tree purple (including all shared ancestors) 8. Using a yellow highlighter or colored pencil, circle the clade that includes all pine trees. 9. Using a orange highlighter or colored pencil, circle the clade that includes all gymnosperms 10. Can you tell…arrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning





