
EBK ORGANIC CHEMISTRY
9th Edition
ISBN: 9780100591318
Author: McMurry
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 29.SE, Problem 18VC
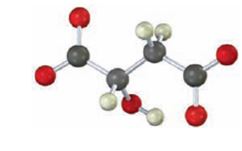
Identify the following intermediate in the citric acid cycle, and tell whether it has R or S stereochemistry:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Predict the major products of this organic reaction:
1. LDA (-78°C)
?
2. Br
Some notes:
• Draw only the major product, or products. You can draw them in any arrangement you like.
.
• Be sure to use wedge and dash bonds where necessary, for example to distinguish between major products that are enantiomers.
• If there are no products, just check the box under the drawing area.
No reaction.
Click and drag to start drawing a structure.
X
Please draw the structures
Draw the missing intermediates 1 and 2, plus the final product 3, of this synthesis:
0
1. Eto
1. Eto-
1
2
2. MeBr
2. EtBr
H3O+
A
3
You can draw the three structures in any arrangement you like.
Explanation
Check
Click and drag to start drawing a structure.
Chapter 29 Solutions
EBK ORGANIC CHEMISTRY
Ch. 29.1 - Prob. 1PCh. 29.3 - Write the equations for the remaining passages of...Ch. 29.3 - Prob. 3PCh. 29.4 - Write a mechanism for the dehydration reaction of...Ch. 29.4 - Evidence for the role of acetate in fatty-acid...Ch. 29.4 - Does the reduction of acetoacetyl ACP in step 6...Ch. 29.5 - Prob. 7PCh. 29.5 - Look at the entire glycolysis pathway, and make a...Ch. 29.6 - Prob. 9PCh. 29.7 - Prob. 10P
Ch. 29.7 - Write mechanisms for step 2 of the citric acid...Ch. 29.7 - Prob. 12PCh. 29.8 - Prob. 13PCh. 29.9 - Write all the steps in the transamination reaction...Ch. 29.9 - What -keto acid is formed on transamination of...Ch. 29.9 - Prob. 16PCh. 29.SE - Prob. 17VCCh. 29.SE - Identify the following intermediate in the citric...Ch. 29.SE - The following compound is an intermediate in the...Ch. 29.SE - Prob. 20VCCh. 29.SE - In the pentose phosphate pathway for degrading...Ch. 29.SE - Prob. 22MPCh. 29.SE - One of the steps in the pentose phosphate pathway...Ch. 29.SE - One of the steps in the pentose phosphate pathway...Ch. 29.SE - Prob. 25MPCh. 29.SE - Prob. 26MPCh. 29.SE - Prob. 27MPCh. 29.SE - Prob. 28MPCh. 29.SE - Prob. 29MPCh. 29.SE - Prob. 30MPCh. 29.SE - Prob. 31MPCh. 29.SE - Prob. 32APCh. 29.SE - Prob. 33APCh. 29.SE - Prob. 34APCh. 29.SE - Prob. 35APCh. 29.SE - Prob. 36APCh. 29.SE - Prob. 37APCh. 29.SE - Prob. 38APCh. 29.SE - Prob. 39APCh. 29.SE - Prob. 40APCh. 29.SE - Prob. 41APCh. 29.SE - Prob. 42APCh. 29.SE - Prob. 43APCh. 29.SE - Prob. 44APCh. 29.SE - Prob. 45APCh. 29.SE - Prob. 46APCh. 29.SE - Prob. 47APCh. 29.SE - Prob. 48APCh. 29.SE - Prob. 49APCh. 29.SE - Prob. 50APCh. 29.SE - In glycerol metabolism, the oxidation of...Ch. 29.SE - Prob. 52APCh. 29.SE - Prob. 53APCh. 29.SE - Prob. 54APCh. 29.SE - In step 7 of fatty-acid biosynthesis (Figure...Ch. 29.SE - Prob. 56AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the missing intermediate 1 and final product 2 of this synthesis: 1. MeO- H3O+ 1 2 2. PrBr Δ You can draw the two structures in any arrangement you like. Click and drag to start drawing a structure.arrow_forwardWhat is the differences between: Glyceride and phosphoglyceride Wax and Fat Soap and Fatty acid HDL and LDL cholesterol Phospho lipids and sphingosine What are the types of lipids? What are the main lipid components of membrane structures? How could lipids play important rules as signaling molecules and building units? The structure variety of lipids makes them to play significant rules in our body, conclude breifly on this statement.arrow_forwardWhat is the differences between DNA and RNA for the following: - structure - function - type What is the meaning of: - replication - transcription - translation show the base pair connection(hydrogen bond) in DNA and RNAarrow_forward
- What is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forwardWhat is the IP for a amino acid- give an example what are the types of amino acids What are the structures of proteins The N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Try-Val-His Sar-Arg-Val His-Pro-Ala Val- Tyr- Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward> aw the missing intermediates 1 and 2, plus the final product 3, of this synthesis: 1. Eto 1. EtO¯ H3O+ 1 2 2. PrBr 2. PrBr Δ You can draw the three structures in any arrangement you like. 3 Click and drag to start drawing a structure. Explanation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacarrow_forward
- There are various factors that affect an equilibrium. Give 3 of these factors and explain using examples andequations how an equilibrium is affected by these factors. Please remember that this is a communication question so that you are communicating your understanding of the factors that affect and equilibrium.arrow_forwardEEZE LETCHUP ID Draw the most likely conjugate base resulting from this acid-base reaction. Include all lone pairs. Ignore inorganic byproducts. Drawing く NaOCH2CH3 :0: :0: 狗arrow_forwardAnswerarrow_forward
- 2. Provide a clear arrow-pushing mechanism for the following reactions. Do not skip proton transfers, do not combine steps, and make sure your arrows are clear enough to be interpreted without ambiguity. a. CH3 Ph OEt هد Ph CH3 Hint: the species on the left is an ynolate, which behaves a lot like an enolate.arrow_forwardb. CH3 H3C CH3 CH3 H3C an unexpected product, containing a single 9- membered ring the expected product, containing two fused rings H3C-I (H3C)2CuLi an enolatearrow_forwardb. H3C CH3 1. 2. H3O+ H3C MgBr H3Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
DIGESTER-35 | VITAMINS AND THEIR RELATED COENZYMES| GPAT | NIPER | PHARMACIST| DI; Author: GPAT DISCUSSION CENTER;https://www.youtube.com/watch?v=CGrdNYmho0s;License: Standard YouTube License, CC-BY