
Concept explainers
Locate the isoprene units in each compound.
a. 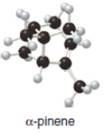 b.
b. 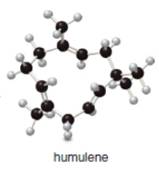
(a)
Interpretation: The isoprene units in the given compound are to be located.
Concept introduction: An isoprene unit possesses following properties.
➢ It may either consist of
➢ It is always connected by one or more
➢ Each isoprene unit is consist of
➢ While locating an isoprene unit, the presence of heteroatoms is ignored.
Answer to Problem 18P
The isoprene units in the given terpene (
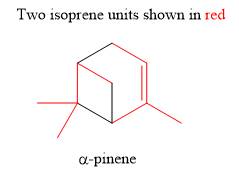
Explanation of Solution
The given compound (
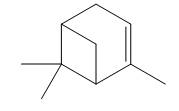
Figure 1
Terpenes are composed of repeating five-carbon units. These units are called isoprene units.
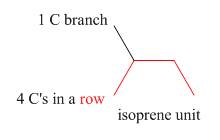
Figure 2
An isoprene unit possesses following properties.
➢ It involves one branched carbon and
➢ It may either consist of
➢ It is always connected by one or more
➢ Each isoprene unit is consist of
➢ While locating an isoprene unit, the presence of heteroatoms is ignored.
The isoprene units in the given terpene (

Figure 3
The isoprene units in the given compound (
(b)
Interpretation: The isoprene units in the given compound are to be located.
Concept introduction: An isoprene unit possesses following properties.
➢ It may either consist of
➢ It is always connected by one or more
➢ Each isoprene unit is consist of
➢ While locating an isoprene unit, the presence of heteroatoms is ignored.
Answer to Problem 18P
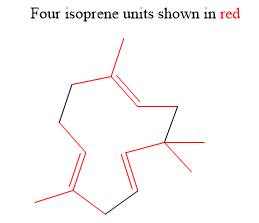
The isoprene units in the given terpene (humulene) are located as,
Explanation of Solution
The given compound (humulene) is a terpene. Its structure is in ball-and stick form. It is converted into skeletal structure by replacing black ball with
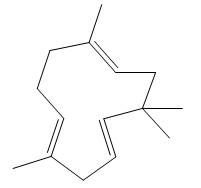
Figure 4
Terpenes are composed of repeating five-carbon units. These units are called isoprene units.

Figure 2
An isoprene unit possesses following properties.
➢ It involves one branched carbon and
➢ It may either consist of
➢ It is always connected by one or more
➢ Each isoprene unit is consist of
➢ While locating an isoprene unit, the presence of heteroatoms is ignored.
The isoprene units in the given terpene (humulene) are located as,

Figure 5
The isoprene units in the given compound (humulene) are located in Figure 5.
Want to see more full solutions like this?
Chapter 29 Solutions
ORGANIC CHEMISTRY
- Identify the compound with the longest carbon - nitrogen bond. O CH3CH2CH=NH O CH3CH2NH2 CH3CH2C=N CH3CH=NCH 3 The length of all the carbon-nitrogen bonds are the samearrow_forwardIdentify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer below. Η H's+ 6Η Η Η Η Η Ηδ Η Ο Ο HH +Η Η +Η Η Η -8+ CIarrow_forwardH H:O::::H H H HH H::O:D:D:H HH HH H:O:D:D:H .. HH H:O:D:D:H H H Select the correct Lewis dot structure for the following compound: CH3CH2OHarrow_forward
- Rank the following compounds in order of decreasing boiling point. ннннн -С-С-Н . н-с- ННННН H ΗΤΗ НННН TTTĪ н-с-с-с-с-о-н НННН НН C' Н н-с-с-с-с-н НН || Ш НННН H-C-C-C-C-N-H ННННН IVarrow_forwardRank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forward
- Why isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forwardWhat is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forward
- Complete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forwardQ Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

